Homologous Recombination and Translesion DNA Synthesis Play Critical Roles on Tolerating DNA Damage Caused by Trace Levels of Hexavalent Chromium
- PMID: 27907204
- PMCID: PMC5132242
- DOI: 10.1371/journal.pone.0167503
Homologous Recombination and Translesion DNA Synthesis Play Critical Roles on Tolerating DNA Damage Caused by Trace Levels of Hexavalent Chromium
Abstract
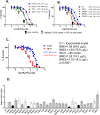
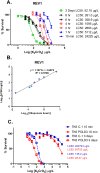
Contamination of potentially carcinogenic hexavalent chromium (Cr(VI)) in the drinking water is a major public health concern worldwide. However, little information is available regarding the biological effects of a nanomoler amount of Cr(VI). Here, we investigated the genotoxic effects of Cr(VI) at nanomoler levels and their repair pathways. We found that DNA damage response analyzed based on differential toxicity of isogenic cells deficient in various DNA repair proteins is observed after a three-day incubation with K2CrO4 in REV1-deficient DT40 cells at 19.2 μg/L or higher as well as in TK6 cells deficient in polymerase delta subunit 3 (POLD3) at 9.8 μg/L or higher. The genotoxicity of Cr(VI) decreased ~3000 times when the incubation time was reduced from three days to ten minutes. TK mutation rate also significantly decreased from 6 day to 1 day exposure to Cr(VI). The DNA damage response analysis suggest that DNA repair pathways, including the homologous recombination and REV1- and POLD3-mediated error-prone translesion synthesis pathways, are critical for the cells to tolerate to DNA damage caused by trace amount of Cr(VI).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms.Mutat Res. 2003 Dec 10;533(1-2):3-36. doi: 10.1016/j.mrfmmm.2003.09.006. Mutat Res. 2003. PMID: 14643411 Review.
-
Intracellular and extracellular factors influencing Cr(VI) and Cr(III) genotoxicity.Environ Mol Mutagen. 2012 Mar;53(2):94-100. doi: 10.1002/em.20679. Epub 2011 Oct 21. Environ Mol Mutagen. 2012. PMID: 22020802
-
A comparison of particulate hexavalent chromium cytotoxicity and genotoxicity in human and leatherback sea turtle lung cells from a one environmental health perspective.Toxicol Appl Pharmacol. 2019 Aug 1;376:70-81. doi: 10.1016/j.taap.2019.05.013. Epub 2019 May 18. Toxicol Appl Pharmacol. 2019. PMID: 31108106 Free PMC article.
-
LncRNA expression profiling and its relationship with DNA damage in Cr(VI)-treated 16HBE cells.Sci Total Environ. 2019 Mar 10;655:622-632. doi: 10.1016/j.scitotenv.2018.11.074. Epub 2018 Nov 8. Sci Total Environ. 2019. PMID: 30476843
-
Effects of hexavalent chromium on mitochondria and their implications in carcinogenesis.J Environ Sci Health C Toxicol Carcinog. 2024;42(2):109-125. doi: 10.1080/26896583.2024.2301899. Epub 2024 Jan 17. J Environ Sci Health C Toxicol Carcinog. 2024. PMID: 38230947 Review.
Cited by
-
Roles of Bacillus subtilis RecA, Nucleotide Excision Repair, and Translesion Synthesis Polymerases in Counteracting Cr(VI)-Promoted DNA Damage.J Bacteriol. 2019 Mar 26;201(8):e00073-19. doi: 10.1128/JB.00073-19. Print 2019 Apr 15. J Bacteriol. 2019. PMID: 30745368 Free PMC article.
-
DNA Repair Molecular Beacon assay: a platform for real-time functional analysis of cellular DNA repair capacity.Oncotarget. 2018 Aug 3;9(60):31719-31743. doi: 10.18632/oncotarget.25859. eCollection 2018 Aug 3. Oncotarget. 2018. PMID: 30167090 Free PMC article.
-
Hexavalent Chromium Exposure Induces Intestinal Barrier Damage via Activation of the NF-κB Signaling Pathway and NLRP3 Inflammasome in Ducks.Front Immunol. 2022 Jul 22;13:952639. doi: 10.3389/fimmu.2022.952639. eCollection 2022. Front Immunol. 2022. PMID: 35935959 Free PMC article.
-
Development of a novel PIG-A gene mutation assay based on a GPI-anchored fluorescent protein sensor.Genes Environ. 2019 Dec 10;41:21. doi: 10.1186/s41021-019-0135-6. eCollection 2019. Genes Environ. 2019. PMID: 31867084 Free PMC article.
-
Tdp1 processes chromate-induced single-strand DNA breaks that collapse replication forks.PLoS Genet. 2018 Aug 27;14(8):e1007595. doi: 10.1371/journal.pgen.1007595. eCollection 2018 Aug. PLoS Genet. 2018. PMID: 30148840 Free PMC article.
References
-
- Anderson RA. Essentiality of chromium in humans. The Science of the total environment. 1989;86(1–2):75–81. - PubMed
-
- Agency for Toxic Substances and Disease Registry Toxicological Profile for Chromium. US Department of Health and Human Services, Washington, DC: 2000.
-
- Liu KJ, Shi X. In vivo reduction of chromium (VI) and its related free radical generation. Mol Cell Biochem. 2001;222(1–2):41–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

