Modern Synthetic Avenues for the Preparation of Functional Fluorophores
- PMID: 27907246
- PMCID: PMC5396271
- DOI: 10.1002/anie.201609394
Modern Synthetic Avenues for the Preparation of Functional Fluorophores
Abstract
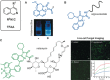
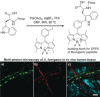
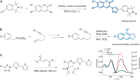
Biomedical research relies on the fast and accurate profiling of specific biomolecules and cells in a non-invasive manner. Functional fluorophores are powerful tools for such studies. As these sophisticated structures are often difficult to access through conventional synthetic strategies, new chemical processes have been developed in the past few years. In this Minireview, we describe the most recent advances in the design, preparation, and fine-tuning of fluorophores by means of multicomponent reactions, C-H activation processes, cycloadditions, and biomolecule-based chemical transformations.
Keywords: C−H activation; fluorescent probes; imaging; microscopy; multicomponent reactions.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




Similar articles
-
Bioorthogonal Chemistry—Introduction and Overview [corrected].Top Curr Chem (Cham). 2016 Feb;374(1):9. doi: 10.1007/s41061-016-0010-x. Epub 2016 Feb 1. Top Curr Chem (Cham). 2016. PMID: 27572992 Review.
-
Combinatorial discovery of fluorescent pharmacophores by multicomponent reactions in droplet arrays.J Am Chem Soc. 2011 Jul 6;133(26):10058-61. doi: 10.1021/ja204016e. Epub 2011 Jun 14. J Am Chem Soc. 2011. PMID: 21644551
-
Advances in the chemistry of small molecule fluorescent probes.Curr Opin Chem Biol. 2011 Dec;15(6):752-9. doi: 10.1016/j.cbpa.2011.10.013. Epub 2011 Nov 10. Curr Opin Chem Biol. 2011. PMID: 22078994 Review.
-
Quinone-based fluorophores for imaging biological processes.Chem Soc Rev. 2018 Jan 2;47(1):12-27. doi: 10.1039/c7cs00553a. Chem Soc Rev. 2018. PMID: 29099127 Review.
-
Constructing New Bioorthogonal Reagents and Reactions.Acc Chem Res. 2018 May 15;51(5):1073-1081. doi: 10.1021/acs.accounts.7b00606. Epub 2018 May 4. Acc Chem Res. 2018. PMID: 29727171 Free PMC article.
Cited by
-
Addition of Isocyanide-Containing Amino Acids to the Genetic Code for Protein Labeling and Activation.ACS Chem Biol. 2019 Dec 20;14(12):2793-2799. doi: 10.1021/acschembio.9b00678. Epub 2019 Nov 14. ACS Chem Biol. 2019. PMID: 31682403 Free PMC article.
-
Probing binding specificity of the sucrose transporter AtSUC2 with fluorescent coumarin glucosides.J Exp Bot. 2018 Apr 27;69(10):2473-2482. doi: 10.1093/jxb/ery075. J Exp Bot. 2018. PMID: 29506213 Free PMC article.
-
Multicomponent synthesis of chromophores - The one-pot approach to functional π-systems.Front Chem. 2023 Mar 17;11:1124209. doi: 10.3389/fchem.2023.1124209. eCollection 2023. Front Chem. 2023. PMID: 37007054 Free PMC article. Review.
-
Assembly of 1H-isoindole derivatives by selective carbon-nitrogen triple bond activation: access to aggregation-induced emission fluorophores for lipid droplet imaging.Chem Sci. 2019 Jun 12;10(29):7076-7081. doi: 10.1039/c9sc01035a. eCollection 2019 Aug 7. Chem Sci. 2019. PMID: 31588275 Free PMC article.
-
Excited-State Intramolecular Proton Transfer Dyes with Dual-State Emission Properties: Concept, Examples and Applications.Molecules. 2022 Apr 10;27(8):2443. doi: 10.3390/molecules27082443. Molecules. 2022. PMID: 35458640 Free PMC article. Review.
References
-
- Wender P. A., Verma V. A., Paxton T. J., Pillow T. H., Acc. Chem. Res. 2008, 41, 40–49. - PubMed
-
- Brown D. G., Boström J., J. Med. Chem. 2016, 59, 4443–4458. - PubMed
-
- For reviews, see:
-
- Multicomponent reactions (Eds: J. Zhu, H. Bienaymé), Wiley-VCH, Weinheim, 2005;
-
- Multicomponent reactions, Vols. 1, 2 (Ed.: T. J. J. Müller), Science of Synthesis, Thieme, Stuttgart, 2014.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

