EGFR kinase inhibitors and gastric acid suppressants in EGFR-mutant NSCLC: a retrospective database analysis of potential drug interaction
- PMID: 27907909
- PMCID: PMC5356756
- DOI: 10.18632/oncotarget.13458
EGFR kinase inhibitors and gastric acid suppressants in EGFR-mutant NSCLC: a retrospective database analysis of potential drug interaction
Abstract
Background: Erlotinib and gefitinib are weak base drugs whose absorption and clinical efficacy may be impaired by concomitant gastric acid suppressive (AS) therapy, yet proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2As) are widely indicated in non-small cell lung cancer (NSCLC) patients for the prevention and treatment of erlotinib-induced gastrointestinal injury and corticosteroid-associated gastric irritation. We assessed the clinical relevance of this potential drug-drug interaction (DDI) in a retrospective cohort of EGFR-mutant NSCLC patients.
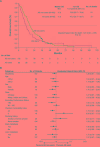
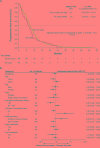
Results: The AS usage rate was 35%. In the overall cohort, AS users did not experience poorer OS (HR: 1.47, 95% CI: 0.92 - 2.35, P = 0.10; median, 11.4 versus 17.5 months) or PFS (HR = 1.37, 95% CI: 0.89 - 2.12, P = 0.16; median, 7.6 versus 8.7 months) compared with non-users in multivariate Cox regression analysis. However, subgroup analyses indicated that AS usage was associated with significantly poorer OS and PFS in patients who had fewer or milder comorbidities (Charlson comorbidity index ≤ 2), those with Karnofsky performance status < 90, and never-smokers.
Materials and methods: A retrospective database analysis of 157 patients given erlotinib or gefitinib for EGFR-mutant advanced NSCLC from two institutions was conducted. Patients were classified as AS-users if the periods of AS and anti-EGFR therapy overlapped by ≥ 30%. Overall survival (OS) and progression-free survival (PFS) were assessed according to AS usage.
Conclusions: Concomitant AS therapy did not have an adverse impact on OS and/or PFS in the overall cohort. Our subgroup findings should be regarded exploratory and require replication in a large prospective cohort.
Keywords: NSCLC; drug-drug interactions; erlotinib; gastric acid suppression; gefitinib.
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures
References
-
- Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai C-M, Tan EH, Ho JC-M, Chu DT, Zaatar A, Osorio Sanchez JA, Vu V Van, Au JSK, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605. doi: 10.1093/jnci/djt072. - DOI - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;2013;11:121–8. doi: 10.1016/S1470-2045(09)70364-X.. - DOI - PubMed
-
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

