Aβ Amyloid Pathology Affects the Hearts of Patients With Alzheimer's Disease: Mind the Heart
- PMID: 27908343
- PMCID: PMC5142757
- DOI: 10.1016/j.jacc.2016.08.073
Aβ Amyloid Pathology Affects the Hearts of Patients With Alzheimer's Disease: Mind the Heart
Abstract
Background: Individually, heart failure (HF) and Alzheimer's disease (AD) are severe threats to population health, and their potential coexistence is an alarming prospect. In addition to sharing analogous epidemiological and genetic profiles, biochemical characteristics, and common triggers, the authors recently recognized common molecular and pathological features between the 2 conditions. Whereas cognitive impairment has been linked to HF through perfusion defects, angiopathy, and inflammation, whether patients with AD present with myocardial dysfunction, and if the 2 conditions bear a common pathogenesis as neglected siblings are unknown.
Objectives: Here, the authors investigated whether amyloid beta (Aβ) protein aggregates are present in the hearts of patients with a primary diagnosis of AD, affecting myocardial function.
Methods: The authors examined myocardial function in a retrospective cross-sectional study from a cohort of AD patients and age-matched controls. Imaging and proteomics approaches were used to identify and quantify Aβ deposits in AD heart and brain specimens compared with controls. Cell shortening and calcium transients were measured on isolated adult cardiomyocytes.
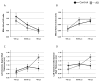
Results: Echocardiographic measurements of myocardial function suggest that patients with AD present with an anticipated diastolic dysfunction. As in the brain, Aβ40 and Aβ42 are present in the heart, and their expression is increased in AD.
Conclusions: Here, the authors provide the first report of the presence of compromised myocardial function and intramyocardial deposits of Aβ in AD patients. The findings depict a novel biological framework in which AD may be viewed either as a systemic disease or as a metastatic disorder leading to heart, and possibly multiorgan failure. AD and HF are both debilitating and life-threatening conditions, affecting enormous patient populations. Our findings underline a previously dismissed problem of a magnitude that will require new diagnostic approaches and treatments for brain and heart disease, and their combination.
Keywords: amyloidosis; cardiomyopathy; dementia; heart failure; protein aggregates.
Copyright © 2016 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures







Comment in
-
The Head and the Heart: The Alzheimer's Connection.J Am Coll Cardiol. 2016 Dec 6;68(22):2408-2411. doi: 10.1016/j.jacc.2016.09.934. J Am Coll Cardiol. 2016. PMID: 27908344 No abstract available.
-
The Head and the Heart: Potential Long-Term Side Effect of ARNI.J Am Coll Cardiol. 2017 Apr 11;69(14):1879-1880. doi: 10.1016/j.jacc.2016.12.043. J Am Coll Cardiol. 2017. PMID: 28385323 No abstract available.
References
-
- National Institute on Aging. [Accessed September 20, 2016];Alzheimer’s Disease Progress Report 2014–2015: Advancing Research Toward a Cure. 2015 Available at: https://www.nia.nih.gov/alzheimers/publication/2014-2015-alzheimers-dise....
-
- Moreira PI, Nunomura A, Honda K, et al. The Key Role of Oxidative Stress in Alzheimer’s Disease. In: Ali Qureshi G, Hassan Parvez S, editors. Oxidative Stress and Neurodegenerative Disorders. Amsterdam, The Netherlands: Elsevier; 2007. pp. 267–82.
-
- Moreira PI, Smith MA, Zhu X, et al. Oxidative stress and neurodegeneration. Ann N Y Acad Sci. 2005;1043:545–52. - PubMed
-
- Tan MS, Yu JT, Tan L. Bridging integrator 1 (BIN1): form, function, and Alzheimer’s disease. Trends Mol Med. 2013;19:594–603. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

