Pim1 Kinase Overexpression Enhances ckit+ Cardiac Stem Cell Cardiac Repair Following Myocardial Infarction in Swine
- PMID: 27908351
- PMCID: PMC5223744
- DOI: 10.1016/j.jacc.2016.09.925
Pim1 Kinase Overexpression Enhances ckit+ Cardiac Stem Cell Cardiac Repair Following Myocardial Infarction in Swine
Abstract
Background: Pim1 kinase plays an important role in cell division, survival, and commitment of precursor cells towards a myocardial lineage, and overexpression of Pim1 in ckit+ cardiac stem cells (CSCs) enhances their cardioreparative properties.
Objectives: The authors sought to validate the effect of Pim1-modified CSCs in a translationally relevant large animal preclinical model of myocardial infarction (MI).
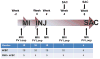
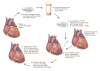
Methods: Human cardiac stem cells (hCSCs, n = 10), hckit+ CSCs overexpressing Pim1 (Pim1+; n = 9), or placebo (n = 10) were delivered by intramyocardial injection to immunosuppressed Yorkshire swine (n = 29) 2 weeks after MI. Cardiac magnetic resonance and pressure volume loops were obtained before and after cell administration.
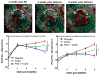
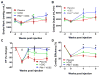
Results: Whereas both hCSCs reduced MI size compared to placebo, Pim1+ cells produced a ∼3-fold greater decrease in scar mass at 8 weeks post-injection compared to hCSCs (-29.2 ± 2.7% vs. -8.4 ± 0.7%; p < 0.003). Pim1+ hCSCs also produced a 2-fold increase of viable mass compared to hCSCs at 8 weeks (113.7 ± 7.2% vs. 65.6 ± 6.8%; p <0.003), and a greater increase in regional contractility in both infarct and border zones (both p < 0.05). Both CSC types significantly increased ejection fraction at 4 weeks but this was only sustained in the Pim1+ group at 8 weeks compared to placebo. Both hCSC and Pim1+ hCSC treatment reduced afterload (p = 0.02 and p = 0.004, respectively). Mechanoenergetic recoupling was significantly greater in the Pim1+ hCSC group (p = 0.005).
Conclusions: Pim1 overexpression enhanced the effect of intramyocardial delivery of CSCs to infarcted porcine hearts. These findings provide a rationale for genetic modification of stem cells and consequent translation to clinical trials.
Keywords: heart failure; human cardiac progenitor cells; injection; pressure volume.
Copyright © 2016 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Pim1 Overexpressing ckit+ Cardiac Stem Cells in Cardiac Regeneration: Preconditioning as Next-Generation Stem Cell Therapy?J Am Coll Cardiol. 2016 Dec 6;68(22):2465-2466. doi: 10.1016/j.jacc.2016.09.924. J Am Coll Cardiol. 2016. PMID: 27908352 No abstract available.
References
-
- Muraski JA, Rota M, Misao Y, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–75. - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL122525/HL/NHLBI NIH HHS/United States
- R01 HL107110/HL/NHLBI NIH HHS/United States
- R01 HL084275/HL/NHLBI NIH HHS/United States
- R01 HL110737/HL/NHLBI NIH HHS/United States
- R37 HL091102/HL/NHLBI NIH HHS/United States
- R01 HL113647/HL/NHLBI NIH HHS/United States
- R01 HL137355/HL/NHLBI NIH HHS/United States
- R01 HL117163/HL/NHLBI NIH HHS/United States
- UM1 HL113460/HL/NHLBI NIH HHS/United States
- R01 HL105759/HL/NHLBI NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- P01 HL085577/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

