The Effectiveness of Web-Based Asthma Self-Management System, My Asthma Portal (MAP): A Pilot Randomized Controlled Trial
- PMID: 27908846
- PMCID: PMC5159614
- DOI: 10.2196/jmir.5866
The Effectiveness of Web-Based Asthma Self-Management System, My Asthma Portal (MAP): A Pilot Randomized Controlled Trial
Abstract
Background: Whether Web-based technologies can improve disease self-management is uncertain. My Asthma Portal (MAP) is a Web-based self-management support system that couples evidence-based behavioral change components (self-monitoring of symptoms, physical activity, and medication adherence) with real-time monitoring, feedback, and support from a nurse case manager.
Objective: The aim of this study was to compare the impact of access to a Web-based asthma self-management patient portal linked to a case-management system (MAP) over 6 months compared with usual care on asthma control and quality of life.
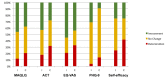
Methods: A multicenter, parallel, 2-arm, pilot, randomized controlled trial was conducted with 100 adults with confirmed diagnosis of asthma from 2 specialty clinics. Asthma control was measured using an algorithm based on overuse of fast-acting bronchodilators and emergency department visits, and asthma-related quality of life was assessed using the Mini-Asthma Quality of Life Questionnaire (MAQLQ). Secondary mediating outcomes included asthma symptoms, depressive symptoms, self-efficacy, and beliefs about medication. Process evaluations were also included.
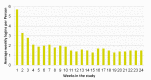
Results: A total of 49 individuals were randomized to MAP and 51 to usual care. Compared with usual care, participants in the intervention group reported significantly higher asthma quality of life (mean change 0.61, 95% CI 0.03 to 1.19), and the change in asthma quality of life for the intervention group between baseline and 3 months (mean change 0.66, 95% CI 0.35 to 0.98) was not seen in the control group. No significant differences in asthma quality of life were found between the intervention and control groups at 6 (mean change 0.46, 95% CI -0.12 to 1.05) and 9 months (mean change 0.39, 95% CI -0.2 to 0.98). For poor control status, there was no significant effect of group, time, or group by time. For all self-reported measures, the intervention group had a significantly higher proportion of individuals, demonstrating a minimal clinically meaningful improvement compared with the usual care group.
Conclusions: This study supported the use of MAP to enhance asthma quality of life but not asthma control as measured by an administrative database. Implementation of MAP beyond 6 months with tailored protocols for monitoring symptoms and health behaviors as individuals' knowledge and self-management skills improve may result in long-term gains in asthma control.
Clinicaltrial: International Standard Randomized Controlled Trial Number (ISRCTN): 34326236; http://www.isrctn.com/ISRCTN34326236 (Archived by Webcite at http://www.webcitation.org/6mGxoI1R7).
Keywords: Internet; asthma; case management; nursing; quality of life; self-care.
©Sara Ahmed, Pierre Ernst, Susan J Bartlett, Marie-France Valois, Tasneem Zaihra, Guy Paré, Roland Grad, Owis Eilayyan, Robert Perreault, Robyn Tamblyn. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 01.12.2016.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Boulet LP, Bai TR, Becker A, Bérubé D, Beveridge R, Bowie DM, Chapman KR, Côté J, Cockcroft D, Ducharme FM, Ernst P, FitzGerald JM, Kovesi T, Hodder RV, O'Byrne P, Rowe B, Sears MR, Simons FE, Spier S. What is new since the last (1999) Canadian Asthma Consensus Guidelines? Can Respir J. 2001;8(Suppl A):5A–27A. - PubMed
-
- Ernst E. Complementary therapies for asthma: what patients use. J Asthma. 1998;35(8):667–71. - PubMed
-
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, Löfdahl CG, Pauwels RA, Ullman A. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999 Aug;160(2):594–9. doi: 10.1164/ajrccm.160.2.9811100. - DOI - PubMed
-
- Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002 Apr;39(2):167–79. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

