A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells
- PMID: 27908912
- PMCID: PMC5834230
- DOI: 10.1161/CIRCRESAHA.116.309782
A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells
Abstract
Rationale: Cardiac progenitor cells are an attractive cell type for tissue regeneration, but their mechanism for myocardial remodeling is still unclear.
Objective: This investigation determines how chronological age influences the phenotypic characteristics and the secretome of human cardiac progenitor cells (CPCs), and their potential to recover injured myocardium.
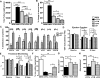
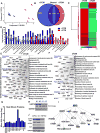
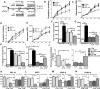
Methods and results: Adult (aCPCs) and neonatal (nCPCs) cells were derived from patients aged >40 years or <1 month, respectively, and their functional potential was determined in a rodent myocardial infarction model. A more robust in vitro proliferative capacity of nCPCs, compared with aCPCs, correlated with significantly greater myocardial recovery mediated by nCPCs in vivo. Strikingly, a single injection of nCPC-derived total conditioned media was significantly more effective than nCPCs, aCPC-derived TCM, or nCPC-derived exosomes in recovering cardiac function, stimulating neovascularization, and promoting myocardial remodeling. High-resolution accurate mass spectrometry with reverse phase liquid chromatography fractionation and mass spectrometry was used to identify proteins in the secretome of aCPCs and nCPCs, and the literature-based networking software identified specific pathways affected by the secretome of CPCs in the setting of myocardial infarction. Examining the TCM, we quantified changes in the expression pattern of 804 proteins in nCPC-derived TCM and 513 proteins in aCPC-derived TCM. The literature-based proteomic network analysis identified that 46 and 6 canonical signaling pathways were significantly targeted by nCPC-derived TCM and aCPC-derived TCM, respectively. One leading candidate pathway is heat-shock factor-1, potentially affecting 8 identified pathways for nCPC-derived TCM but none for aCPC-derived TCM. To validate this prediction, we demonstrated that the modulation of heat-shock factor-1 by knockdown in nCPCs or overexpression in aCPCs significantly altered the quality of their secretome.
Conclusions: A deep proteomic analysis revealed both detailed and global mechanisms underlying the chronological age-based differences in the ability of CPCs to promote myocardial recovery via the components of their secretome.
Keywords: adult stem cells; exosomes; heat-shock proteins; myocardial ischemia; proteomics; stem cells.
© 2016 American Heart Association, Inc.
Figures
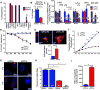
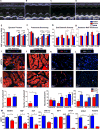
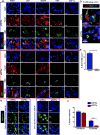
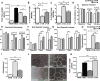
Comment in
-
Young Hearts Run Free: Therapeutic Potential of Neonatal Human Cardiac Progenitor Cells Secretome.Circ Res. 2017 Mar 3;120(5):751-752. doi: 10.1161/CIRCRESAHA.117.310574. Circ Res. 2017. PMID: 28254793 Free PMC article. No abstract available.
Similar articles
-
Cardiosphere-derived cells from pediatric end-stage heart failure patients have enhanced functional activity due to the heat shock response regulating the secretome.Stem Cells. 2015 Apr;33(4):1213-29. doi: 10.1002/stem.1937. Stem Cells. 2015. PMID: 25752510 Free PMC article.
-
Cell-based therapies reverse the heart failure-altered right ventricular proteome towards a pre-disease state.Stem Cell Res Ther. 2024 Nov 13;15(1):420. doi: 10.1186/s13287-024-04009-3. Stem Cell Res Ther. 2024. PMID: 39533351 Free PMC article.
-
Autologous Cardiac Stem Cell Injection in Patients with Hypoplastic Left Heart Syndrome (CHILD Study).Pediatr Cardiol. 2022 Oct;43(7):1481-1493. doi: 10.1007/s00246-022-02872-6. Epub 2022 Apr 8. Pediatr Cardiol. 2022. PMID: 35394149
-
Stem cells: insights into the secretome.Biochim Biophys Acta. 2013 Nov;1834(11):2380-4. doi: 10.1016/j.bbapap.2013.01.032. Epub 2013 Jan 31. Biochim Biophys Acta. 2013. PMID: 23376432 Review.
-
[Stem cells after myocardial infarction].Herz. 2006 Apr;31(2):127-36; quiz 142-3. doi: 10.1007/s00059-006-2794-x. Herz. 2006. PMID: 16738836 Review. German.
Cited by
-
The Multifunctional Contribution of FGF Signaling to Cardiac Development, Homeostasis, Disease and Repair.Front Cell Dev Biol. 2021 May 14;9:672935. doi: 10.3389/fcell.2021.672935. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095143 Free PMC article. Review.
-
Extracellular vesicle interplay in cardiovascular pathophysiology.Am J Physiol Heart Circ Physiol. 2021 May 1;320(5):H1749-H1761. doi: 10.1152/ajpheart.00925.2020. Epub 2021 Mar 5. Am J Physiol Heart Circ Physiol. 2021. PMID: 33666501 Free PMC article. Review.
-
Extracellular Vesicles in Cardiovascular Theranostics.Theranostics. 2017 Sep 26;7(17):4168-4182. doi: 10.7150/thno.21274. eCollection 2017. Theranostics. 2017. PMID: 29158817 Free PMC article. Review.
-
Cardiac Progenitor Cell-Derived Extracellular Vesicles Reduce Infarct Size and Associate with Increased Cardiovascular Cell Proliferation.J Cardiovasc Transl Res. 2019 Feb;12(1):5-17. doi: 10.1007/s12265-018-9842-9. Epub 2018 Nov 19. J Cardiovasc Transl Res. 2019. PMID: 30456736 Free PMC article.
-
Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics.Mol Ther. 2018 Jul 5;26(7):1635-1643. doi: 10.1016/j.ymthe.2018.04.024. Epub 2018 May 3. Mol Ther. 2018. PMID: 29807783 Free PMC article. Review.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Executive Summary: Heart Disease and Stroke Statistics–2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–54. - PubMed
-
- Crisostomo V, Casado JG, Baez-Diaz C, Blazquez R, Sanchez-Margallo FM. Allogeneic cardiac stem cell administration for acute myocardial infarction. Expert review of cardiovascular therapy. 2015;13:285–99. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

