The Hypothalamic Glucagon-Like Peptide 1 Receptor Is Sufficient but Not Necessary for the Regulation of Energy Balance and Glucose Homeostasis in Mice
- PMID: 27908915
- PMCID: PMC5248999
- DOI: 10.2337/db16-1102
The Hypothalamic Glucagon-Like Peptide 1 Receptor Is Sufficient but Not Necessary for the Regulation of Energy Balance and Glucose Homeostasis in Mice
Abstract
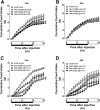
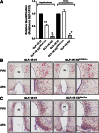
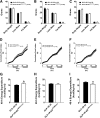
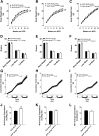
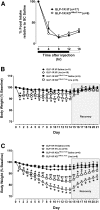
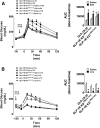
Pharmacological activation of the hypothalamic glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) promotes weight loss and improves glucose tolerance. This demonstrates that the hypothalamic GLP-1R is sufficient but does not show whether it is necessary for the effects of exogenous GLP-1R agonists (GLP-1RA) or endogenous GLP-1 on these parameters. To address this, we crossed mice harboring floxed Glp1r alleles to mice expressing Nkx2.1-Cre to knock down Glp1r expression throughout the hypothalamus (GLP-1RKDΔNkx2.1cre). We also generated mice lacking Glp1r expression specifically in two GLP-1RA-responsive hypothalamic feeding nuclei/cell types, the paraventricular nucleus (GLP-1RKDΔSim1cre) and proopiomelanocortin neurons (GLP-1RKDΔPOMCcre). Chow-fed GLP-1RKDΔNkx2.1cre mice exhibited increased food intake and energy expenditure with no net effect on body weight. When fed a high-fat diet, these mice exhibited normal food intake but elevated energy expenditure, yielding reduced weight gain. None of these phenotypes were observed in GLP-1RKDΔSim1cre and GLP-1RKDΔPOMCcre mice. The acute anorectic and glucose tolerance effects of peripherally dosed GLP-1RA exendin-4 and liraglutide were preserved in all mouse lines. Chronic liraglutide treatment reduced body weight in chow-fed GLP-1RKDΔNkx2.1cre mice, but this effect was attenuated with high-fat diet feeding. In sum, classic homeostatic control regions are sufficient but not individually necessary for the effects of GLP-1RA on nutrient homeostasis.
© 2017 by the American Diabetes Association.
Figures


References
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 - PubMed
-
- Fonseca VA, Zinman B, Nauck MA, Goldfine AB, Plutzky J. Confronting the type 2 diabetes epidemic: the emerging role of incretin-based therapies. Am J Med 2010;123:S2–S10 - PubMed
-
- Burmeister MA, Ayala J, Drucker DJ, Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am J Physiol Endocrinol Metab 2013;304:E677–E685 - PubMed
-
- Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 2003;284:R1427–R1435 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

