Identification of a Substrate-selective Exosite within the Metalloproteinase Anthrax Lethal Factor
- PMID: 27909054
- PMCID: PMC5247655
- DOI: 10.1074/jbc.M116.761734
Identification of a Substrate-selective Exosite within the Metalloproteinase Anthrax Lethal Factor
Abstract
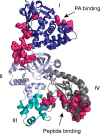
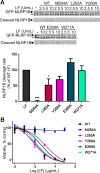
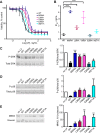
The metalloproteinase anthrax lethal factor (LF) is secreted by Bacillus anthracis to promote disease virulence through disruption of host signaling pathways. LF is a highly specific protease, exclusively cleaving mitogen-activated protein kinase kinases (MKKs) and rodent NLRP1B (NACHT leucine-rich repeat and pyrin domain-containing protein 1B). How LF achieves such restricted substrate specificity is not understood. Previous studies have suggested the existence of an exosite interaction between LF and MKKs that promotes cleavage efficiency and specificity. Through a combination of in silico prediction and site-directed mutagenesis, we have mapped an exosite to a non-catalytic region of LF. Mutations within this site selectively impair proteolysis of full-length MKKs yet have no impact on cleavage of short peptide substrates. Although this region appears important for cleaving all LF protein substrates, we found that mutation of specific residues within the exosite differentially affects MKK and NLRP1B cleavage in vitro and in cultured cells. One residue in particular, Trp-271, is essential for cleavage of MKK3, MKK4, and MKK6 but dispensable for targeting of MEK1, MEK2, and NLRP1B. Analysis of chimeric substrates suggests that this residue interacts with the MKK catalytic domain. We found that LF-W271A blocked ERK phosphorylation and growth in a melanoma cell line, suggesting that it may provide a highly selective inhibitor of MEK1/2 for use as a cancer therapeutic. These findings provide insight into how a bacterial toxin functions to specifically impair host signaling pathways and suggest a general strategy for mapping protease exosite interactions.
Keywords: NACHT leucine-rich repeat and pyrin domain containing protein (NLRP); anthrax toxin; bacterial pathogenesis; host-pathogen interaction; macrophage; melanoma; mitogen-activated protein kinase kinase; protein kinase; proteinase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Alouf J. E. (2000) Bacterial protein toxins: an overview. Methods Mol. Biol. 145, 1–26 - PubMed
-
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., and Jahn R. (1993) Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365, 160–163 - PubMed
-
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino deLaureto P., DasGupta B. R., and Montecucco C. (1992) Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359, 832–835 - PubMed
-
- Turk B. E. (2007) Manipulation of host signalling pathways by anthrax toxins. Biochem. J. 402, 405–417 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

