Superior survival of ex vivo cultured human reticulocytes following transfusion into mice
- PMID: 27909219
- PMCID: PMC5394952
- DOI: 10.3324/haematol.2016.154443
Superior survival of ex vivo cultured human reticulocytes following transfusion into mice
Abstract
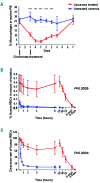
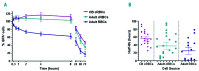
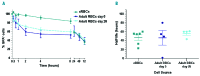
The generation of cultured red blood cells from stem cell sources may fill an unmet clinical need for transfusion-dependent patients, particularly in countries that lack a sufficient and safe blood supply. Cultured red blood cells were generated from human CD34+ cells from adult peripheral blood or cord blood by ex vivo expansion, and a comprehensive in vivo survival comparison with standard red cell concentrates was undertaken. Significant amplification (>105-fold) was achieved using CD34+ cells from both cord blood and peripheral blood, generating high yields of enucleated cultured red blood cells. Following transfusion, higher levels of cultured red cells could be detected in the murine circulation compared to standard adult red cells. The proportions of cultured blood cells from cord or peripheral blood sources remained high 24 hours post-transfusion (82±5% and 78±9%, respectively), while standard adult blood cells declined rapidly to only 49±9% by this time. In addition, the survival time of cultured blood cells in mice was longer than that of standard adult red cells. A paired comparison of cultured blood cells and standard adult red blood cells from the same donor confirmed the enhanced in vivo survival capacity of the cultured cells. The study herein represents the first demonstration that ex vivo generated cultured red blood cells survive longer than donor red cells using an in vivo model that more closely mimics clinical transfusion. Cultured red blood cells may offer advantages for transfusion-dependent patients by reducing the number of transfusions required.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- World Health Organization Global Database on Blood Safety Summary Report. Available from: http://www.who.int/bloodsafety/global_database/GDBS_Summary_Report_2011.... 2011. Last accessed: 29th November 2017.
-
- Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet. 2013;381(9880):1866–1875. - PubMed
-
- Anstee DJ, Gampel A, Toye AM. Ex-vivo generation of human red cells for transfusion. Curr Opin Hematol. 2012;19(3):163–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

