Copper(I)-Dioxygen Adducts and Copper Enzyme Mechanisms
- PMID: 27909346
- PMCID: PMC5125784
- DOI: 10.1002/ijch.201600025
Copper(I)-Dioxygen Adducts and Copper Enzyme Mechanisms
Abstract
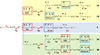
Primary copper(I)-dioxygen (O2) adducts, cupric-superoxide complexes, have been proposed intermediates in copper-containing dioxygen-activating monooxygenase and oxidase enzymes. Here, mechanisms of C-H activation by reactive copper-(di)oxygen intermediates are discussed, with an emphasis on cupric-superoxide species. Over the past 25 years, many synthetically derived cupric-superoxide model complexes have been reported. Due to the thermal instability of these intermediates, early studies focused on increasing their stability and obtaining physical characterization. More recently, in an effort to gain insight into the possible substrate oxidation step in some copper monooxygenases, several cupric-superoxide complexes have been used as surrogates to probe substrate scope and reaction mechanisms. These cupric superoxides are capable of oxidizing substrates containing weak O-H and C-H bonds. Mechanistic studies for some enzymes and model systems have supported an initial hydrogen-atom abstraction via the cupric-superoxide complex as the first step of substrate oxidation.
Keywords: copper; cupric superoxide; dioxygen activation; enzymes; monooxygenase mechanisms.
Figures











References
-
- Theil E, Raymond K. In: Bioinorganic Chemistry. Bertini I, Gray HB, Lippard SJ, Valentine JS, editors. Mill Valley: University Science Books; 1994. pp. 1–35.
-
- Kaila VR, Verkhovsky MI, Wikstrçm M. Chem. Rev. 2010;110:7062–7081. - PubMed
-
- Peterson RL, Kim S, Karlin KD. In: Comprehensive Inorganic Chemistry II: From Elements to Applications. 2nd. Reedijk J, Poeppelmeier K, editors. Oxford: Elsevier; 2013. pp. 149–177.
-
- Garcia-Bosch I, Karlin KD. In: The Chemistry of Peroxides. Greer A, Liebman JF, editors. Vol. 3. Chichester: John Wiley & Sons, Ltd; 2014. pp. 805–856.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
