The Eye As a Biomarker for Alzheimer's Disease
- PMID: 27909396
- PMCID: PMC5112261
- DOI: 10.3389/fnins.2016.00536
The Eye As a Biomarker for Alzheimer's Disease
Abstract
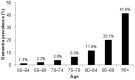
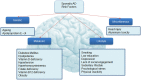
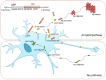
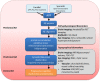
Alzheimer's disease (AD) is a progressive neurodegenerative disorder resulting in dementia and eventual death. It is the leading cause of dementia and the number of cases are projected to rise in the next few decades. Pathological hallmarks of AD include the presence of hyperphosphorylated tau and amyloid protein deposition. Currently, these pathological biomarkers are detected either through cerebrospinal fluid analysis, brain imaging or post-mortem. Though effective, these methods are not widely available due to issues such as the difficulty in acquiring samples, lack of infrastructure or high cost. Given that the eye possesses clear optics and shares many neural and vascular similarities to the brain, it offers a direct window to cerebral pathology. These unique characteristics lend itself to being a relatively inexpensive biomarker for AD which carries the potential for wide implementation. The development of ocular biomarkers can have far implications in the discovery of treatments which can improve the quality of lives of patients. In this review, we consider the current evidence for ocular biomarkers in AD and explore potential future avenues of research in this area.
Keywords: Alzheimer's disease; biomarker; eye; neurodegeneration; ocular; retina.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

