Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation
- PMID: 27909435
- PMCID: PMC5112259
- DOI: 10.3389/fimmu.2016.00507
Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
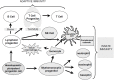
The timely reconstitution and regain of function of a donor-derived immune system is of utmost importance for the recovery and long-term survival of patients after allogeneic hematopoietic stem cell transplantation (HSCT). Of note, new developments such as umbilical cord blood or haploidentical grafts were associated with prolonged immunodeficiency due to delayed immune reconstitution, raising the need for better understanding and enhancing the process of immune reconstitution and finding strategies to further optimize these transplant procedures. Immune reconstitution post-HSCT occurs in several phases, innate immunity being the first to regain function. The slow T cell reconstitution is regarded as primarily responsible for deleterious infections with latent viruses or fungi, occurrence of graft-versus-host disease, and relapse. Here we aim to summarize the major steps of the adaptive immune reconstitution and will discuss the importance of immune balance in patients after HSCT.
Keywords: graft-versus-host disease; graft-versus-leukemia effect; hematopoietic stem cell transplantation; immune reconstitution; infection.
Figures



References
-
- Martin PS, Li S, Nikiforow S, Alyea EP, Antin JH, Armand P, et al. Infused total nucleated cell dose is a better predictor of transplant outcomes than CD34(+) cell number in reduced-intensity mobilized peripheral blood allogeneic hematopoietic cell transplantation. Haematologica (2016) 101(4):499–505. 10.3324/haematol.2015.134841 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

