High-valent copper in biomimetic and biological oxidations
- PMID: 27909921
- PMCID: PMC6013080
- DOI: 10.1007/s00775-016-1420-5
High-valent copper in biomimetic and biological oxidations
Abstract
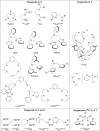
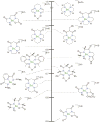
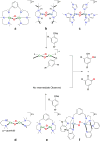
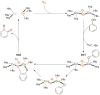
A long-standing debate in the Cu-O2 field has revolved around the relevance of the Cu(III) oxidation state in biological redox processes. The proposal of Cu(III) in biology is generally challenged as no spectroscopic or structural evidence exists currently for its presence. The reaction of synthetic Cu(I) complexes with O2 at low temperature in aprotic solvents provides the opportunity to investigate and define the chemical landscape of Cu-O2 species at a small-molecule level of detail; eight different types are characterized structurally, three of which contain at least one Cu(III) center. Simple imidazole or histamine ligands are competent in these oxygenation reactions to form Cu(III) complexes. The combination of synthetic structural and reactivity data suggests (1) that Cu(I) should be considered as either a one or two electron reductant reacting with O2, (2) that Cu(III) reduction potentials of these formed complexes are modest and well within the limits of a protein matrix and (3) that primary amine and imidazole ligands are surprisingly good at stabilizing Cu(III) centers. These Cu(III) complexes are efficient oxidants for hydroxylating phenolate substrates with reaction hallmarks similar to that performed in biological systems. The remarkable ligation similarity of the synthetic and biological systems makes it difficult to continue to exclude Cu(III) from biological discussions.
Keywords: Biomimetic; Copper(III); Dioxygen activation; Tyrosinase.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

