Melanosome transfer to keratinocyte in the chicken embryonic skin is mediated by vesicle release associated with Rho-regulated membrane blebbing
- PMID: 27910904
- PMCID: PMC5133614
- DOI: 10.1038/srep38277
Melanosome transfer to keratinocyte in the chicken embryonic skin is mediated by vesicle release associated with Rho-regulated membrane blebbing
Abstract
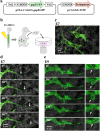
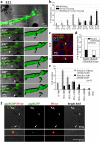
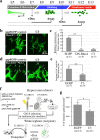
During skin pigmentation in amniotes, melanin synthesized in the melanocyte is transferred to keratinocytes by a particle called the melanosome. Previous studies, mostly using dissociated cultured cells, have proposed several different models that explain how the melanosome transfer is achieved. Here, using a technique that labels the plasma membrane of melanocytes within a three-dimensional system that mimics natural tissues, we have visualized the plasma membrane of melanocytes with EGFP in chicken embryonic skin. Confocal time-lapse microscopy reveals that the melanosome transfer is mediated, at least in part, by vesicles produced by plasma membrane. Unexpectedly, the vesicle release is accompanied by the membrane blebbing of melanocytes. Blebs that have encapsulated a melanosome are pinched off to become vesicles, and these melanosome-containing vesicles are finally engulfed by neighboring keratinocytes. For both the membrane blebbing and vesicle release, Rho small GTPase is essential. We further show that the membrane vesicle-mediated melanosome transfer plays a significant role in the skin pigmentation. Given that the skin pigmentation in inter-feather spaces in chickens is similar to that in inter-hair spaces of humans, our findings should have important consequences in cosmetic medicine.
Figures

Similar articles
-
Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes.Pigment Cell Melanoma Res. 2020 May;33(3):403-415. doi: 10.1111/pcmr.12838. Epub 2019 Nov 11. Pigment Cell Melanoma Res. 2020. PMID: 31659857
-
M-INK, a novel tool for visualizing melanosomes and melanocores.J Biochem. 2017 Apr 1;161(4):323-326. doi: 10.1093/jb/mvw100. J Biochem. 2017. PMID: 28096452
-
Inhibition of melanosome transfer from melanocytes to keratinocytes by lectins and neoglycoproteins in an in vitro model system.Pigment Cell Res. 2001 Jun;14(3):185-94. doi: 10.1034/j.1600-0749.2001.140308.x. Pigment Cell Res. 2001. PMID: 11434566
-
Wide coverage of the body surface by melanocyte-mediated skin pigmentation.Dev Biol. 2019 May 15;449(2):83-89. doi: 10.1016/j.ydbio.2018.04.016. Epub 2018 Apr 23. Dev Biol. 2019. PMID: 29698617 Review.
-
Recent advances in understanding the molecular basis of melanogenesis in melanocytes.F1000Res. 2020 Jun 15;9:F1000 Faculty Rev-608. doi: 10.12688/f1000research.24625.1. eCollection 2020. F1000Res. 2020. PMID: 32595944 Free PMC article. Review.
Cited by
-
Cell Junction and Vesicle Trafficking-Mediated Melanosome/Melanin Transfer Are Involved in the Dynamic Transformation of Goldfish Carassius auratus Skin Color.Int J Mol Sci. 2022 Oct 13;23(20):12214. doi: 10.3390/ijms232012214. Int J Mol Sci. 2022. PMID: 36293071 Free PMC article.
-
Exosome-derived microRNAs: emerging players in vitiligo.Front Immunol. 2024 Jul 8;15:1419660. doi: 10.3389/fimmu.2024.1419660. eCollection 2024. Front Immunol. 2024. PMID: 39040109 Free PMC article. Review.
-
Melanin Transfer and Fate within Keratinocytes in Human Skin Pigmentation.Integr Comp Biol. 2021 Oct 14;61(4):1546-1555. doi: 10.1093/icb/icab094. Integr Comp Biol. 2021. PMID: 34021340 Free PMC article.
-
Shining a Light on Black Holes in Keratinocytes.J Invest Dermatol. 2018 Mar;138(3):486-489. doi: 10.1016/j.jid.2017.11.002. J Invest Dermatol. 2018. PMID: 29477191 Free PMC article.
-
Dermal Fibroblasts Internalize Phosphatidylserine-Exposed Secretory Melanosome Clusters and Apoptotic Melanocytes.Int J Mol Sci. 2020 Aug 12;21(16):5789. doi: 10.3390/ijms21165789. Int J Mol Sci. 2020. PMID: 32806720 Free PMC article.
References
-
- Wu X. & Hammer J. A. 3rd. Making sense of melanosome dynamics in mouse melanocytes. Pigment cell research/sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society 13, 241–247 (2000). - PubMed
-
- Lambert J., Vancoillie G. & Naeyaert J. M. Molecular motors and their role in pigmentation. Cellular and molecular biology (Noisy-le-Grand, France) 45, 905–918 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

