Ascl3 transcription factor marks a distinct progenitor lineage for non-neuronal support cells in the olfactory epithelium
- PMID: 27910949
- PMCID: PMC5133605
- DOI: 10.1038/srep38199
Ascl3 transcription factor marks a distinct progenitor lineage for non-neuronal support cells in the olfactory epithelium
Abstract
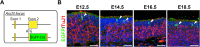
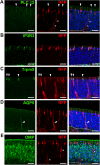
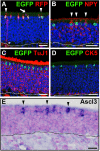
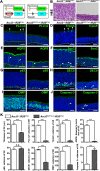
The olfactory epithelium (OE) is composed of olfactory sensory neurons (OSNs), sustentacular supporting cells, and several types of non-neuronal cells. Stem and progenitor cells are located basally, and are the source of all cell types needed to maintain OE homeostasis. Here, we report that Ascl3, a basic helix-loop-helix transcription factor, is expressed in the developing OE. Lineage tracing experiments demonstrate that the non-neuronal microvillar cells and Bowman's glands are exclusively derived from Ascl3+ progenitor cells in the OE during development. Following chemically-induced injury, Ascl3 expression is activated in a subset of horizontal basal cells (HBCs), which repopulate all microvillar cells and Bowman's glands during OE regeneration. After ablation of Ascl3-expressing cells, the OE can regenerate, but lacks the non-neuronal microvillar and Bowman's gland support cells. These results demonstrate that Ascl3 marks progenitors that are lineage-committed strictly to microvillar cells and Bowman's glands, and highlight the requirement for these cell types to support OE homeostasis.
Figures

Similar articles
-
Ascl3 marks adult progenitor cells of the mouse salivary gland.Stem Cell Res. 2012 May;8(3):379-87. doi: 10.1016/j.scr.2012.01.002. Epub 2012 Jan 31. Stem Cell Res. 2012. PMID: 22370009 Free PMC article.
-
Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration.Dev Biol. 2011 May 15;353(2):186-93. doi: 10.1016/j.ydbio.2011.02.025. Epub 2011 Mar 4. Dev Biol. 2011. PMID: 21377457 Free PMC article.
-
Primary Cilia on Horizontal Basal Cells Regulate Regeneration of the Olfactory Epithelium.J Neurosci. 2015 Oct 7;35(40):13761-72. doi: 10.1523/JNEUROSCI.1708-15.2015. J Neurosci. 2015. PMID: 26446227 Free PMC article.
-
Identification and molecular regulation of neural stem cells in the olfactory epithelium.Exp Cell Res. 2005 Jun 10;306(2):309-16. doi: 10.1016/j.yexcr.2005.03.027. Epub 2005 Apr 21. Exp Cell Res. 2005. PMID: 15925585 Review.
-
Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology.J Mol Histol. 2007 Dec;38(6):581-99. doi: 10.1007/s10735-007-9141-2. Epub 2007 Sep 13. J Mol Histol. 2007. PMID: 17851769 Review.
Cited by
-
TRPM5-expressing Microvillous Cells Regulate Region-specific Cell Proliferation and Apoptosis During Chemical Exposure.Neuroscience. 2020 May 10;434:171-190. doi: 10.1016/j.neuroscience.2020.03.029. Epub 2020 Mar 26. Neuroscience. 2020. PMID: 32224228 Free PMC article.
-
Increased Retinoic Acid Catabolism in Olfactory Sensory Neurons Activates Dormant Tissue-Specific Stem Cells and Accelerates Age-Related Metaplasia.J Neurosci. 2020 May 20;40(21):4116-4129. doi: 10.1523/JNEUROSCI.2468-19.2020. Epub 2020 May 8. J Neurosci. 2020. PMID: 32385093 Free PMC article.
-
Salivary gland stem cells: A review of development, regeneration and cancer.Genesis. 2018 May;56(5):e23211. doi: 10.1002/dvg.23211. Epub 2018 May 4. Genesis. 2018. PMID: 29663717 Free PMC article. Review.
-
Cyclophosphamide has Long-Term Effects on Proliferation in Olfactory Epithelia.Chem Senses. 2020 Mar 25;45(2):97-109. doi: 10.1093/chemse/bjz075. Chem Senses. 2020. PMID: 31844905 Free PMC article.
-
Zebrafish olfactory receptors ORAs differentially detect bile acids and bile salts.J Biol Chem. 2019 Apr 26;294(17):6762-6771. doi: 10.1074/jbc.RA118.006483. Epub 2019 Mar 4. J Biol Chem. 2019. PMID: 30833327 Free PMC article.
References
-
- Whitby-Logan G. K., Weech M. & Walters E. Zonal expression and activity of glutathione S-transferase enzymes in the mouse olfactory mucosa. Brain Res 995, 151–7 (2004). - PubMed
-
- Holbrook E. H., Szumowski K. E. & Schwob J. E. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol 363, 129–46 (1995). - PubMed
-
- Solbu T. T. & Holen T. Aquaporin Pathways and Mucin Secretion of Bowman’s Glands Might Protect the Olfactory Mucosa. Chemical Senses (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

