Secretory IgA Deficiency in Individual Small Airways Is Associated with Persistent Inflammation and Remodeling
- PMID: 27911098
- PMCID: PMC5422646
- DOI: 10.1164/rccm.201604-0759OC
Secretory IgA Deficiency in Individual Small Airways Is Associated with Persistent Inflammation and Remodeling
Abstract
Rationale: Maintenance of a surface immune barrier is important for homeostasis in organs with mucosal surfaces that interface with the external environment; however, the role of the mucosal immune system in chronic lung diseases is incompletely understood.
Objectives: We examined the relationship between secretory IgA (SIgA) on the mucosal surface of small airways and parameters of inflammation and airway wall remodeling in chronic obstructive pulmonary disease (COPD).
Methods: We studied 1,104 small airways (<2 mm in diameter) from 50 former smokers with COPD and 39 control subjects. Small airways were identified on serial tissue sections and examined for epithelial morphology, SIgA, bacterial DNA, nuclear factor-κB activation, neutrophil and macrophage infiltration, and airway wall thickness.
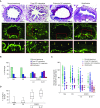
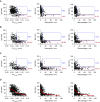
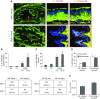
Measurements and main results: Morphometric evaluation of small airways revealed increased mean airway wall thickness and inflammatory cell counts in lungs from patients with COPD compared with control subjects, whereas SIgA level on the mucosal surface was decreased. However, when small airways were classified as SIgA intact or SIgA deficient, we found that pathologic changes were localized almost exclusively to SIgA-deficient airways, regardless of study group. SIgA-deficient airways were characterized by (1) abnormal epithelial morphology, (2) invasion of bacteria across the apical epithelial barrier, (3) nuclear factor-κB activation, (4) accumulation of macrophages and neutrophils, and (5) fibrotic remodeling of the airway wall.
Conclusions: Our findings support the concept that localized, acquired SIgA deficiency in individual small airways of patients with COPD allows colonizing bacteria to cross the epithelial barrier and drive persistent inflammation and airway wall remodeling, even after smoking cessation.
Keywords: NF-κB; chronic obstructive pulmonary disease; neutrophils; secretory IgA; small airways.
Figures


Comment in
-
A Hairline Crack in the Levee: Focal Secretory IgA Deficiency as a First Step toward Emphysema.Am J Respir Crit Care Med. 2017 Apr 15;195(8):970-973. doi: 10.1164/rccm.201612-2509ED. Am J Respir Crit Care Med. 2017. PMID: 28409678 Free PMC article. No abstract available.
References
-
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. - PubMed
-
- 2014 Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Revised 2014. Global Initiative for Chronic Obstructive Lung Disease (GOLD) [accessed 2014 Jan 23]. Available from: www.goldcopd.org.
-
- Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, MacNee W, Make BJ, Rennard SI, Stockley RA, et al. ATS/ERS Task Force for COPD Research. An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:e4–e27. - PubMed
-
- Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. - PubMed
-
- Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol (1985) 1992;72:1016–1023. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

