Mitochondria and Iron: current questions
- PMID: 27911100
- PMCID: PMC5538026
- DOI: 10.1080/17474086.2016.1268047
Mitochondria and Iron: current questions
Erratum in
-
Erratum.Expert Rev Hematol. 2017 Mar;10(3):275. doi: 10.1080/17474086.2017.1287270. Epub 2017 Feb 8. Expert Rev Hematol. 2017. PMID: 28245742 No abstract available.
Abstract
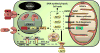
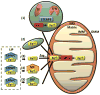
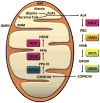
Mitochondria are cellular organelles that perform numerous bioenergetic, biosynthetic, and regulatory functions and play a central role in iron metabolism. Extracellular iron is taken up by cells and transported to the mitochondria, where it is utilized for synthesis of cofactors essential to the function of enzymes involved in oxidation-reduction reactions, DNA synthesis and repair, and a variety of other cellular processes. Areas covered: This article reviews the trafficking of iron to the mitochondria and normal mitochondrial iron metabolism, including heme synthesis and iron-sulfur cluster biogenesis. Much of our understanding of mitochondrial iron metabolism has been revealed by pathologies that disrupt normal iron metabolism. These conditions affect not only iron metabolism but mitochondrial function and systemic health. Therefore, this article also discusses these pathologies, including conditions of systemic and mitochondrial iron dysregulation as well as cancer. Literature covering these areas was identified via PubMed searches using keywords: Iron, mitochondria, Heme Synthesis, Iron-sulfur Cluster, and Cancer. References cited by publications retrieved using this search strategy were also consulted. Expert commentary: While much has been learned about mitochondrial and its iron, key questions remain. Developing a better understanding of mitochondrial iron and its regulation will be paramount in developing therapies for syndromes that affect mitochondrial iron.
Keywords: Iron; cancer; heme synthesis; iron trafficking; iron-sulfur cluster; mitochondria.
Conflict of interest statement
DH Manz received grant T90 DE021989, SV Torti has received grant R01 CA188025, and FM Torti has received grant R01 CA171101, from the US National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467(7318):929–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
