Excitation-Contraction Coupling Alterations in Myopathies
- PMID: 27911331
- PMCID: PMC5240595
- DOI: 10.3233/JND-160172
Excitation-Contraction Coupling Alterations in Myopathies
Abstract
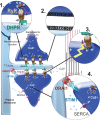
During the complex series of events leading to muscle contraction, the initial electric signal coming from motor neurons is transformed into an increase in calcium concentration that triggers sliding of myofibrils. This process, referred to as excitation-contraction coupling, is reliant upon the calcium-release complex, which is restricted spatially to a sub-compartment of muscle cells ("the triad") and regulated precisely. Any dysfunction in the calcium-release complex leads to muscle impairment and myopathy. Various causes can lead to alterations in excitation-contraction coupling and to muscle diseases. The latter are reviewed and classified into four categories: (i) mutation in a protein of the calcium-release complex; (ii) alteration in triad structure; (iii) modification of regulation of channels; (iv) modification in calcium stores within the muscle. Current knowledge of the pathophysiologic mechanisms in each category is described and discussed.
Keywords: DHPR; RyR1; calcium; congenital myopathies; sarcoplasmic reticulum; triad.
Figures

References
-
- Franzini-Armstrong C and Nunzi G. Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil. 1983;4:233–52. - PubMed
-
- Efremov RG, Leitner A, Aebersold R and Raunser S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature. 2015;517:39–43. doi: 10.1038/nature13916 - DOI - PubMed
-
- Yan Z, Bai XC, Yan C, Wu J, Li Z, Xie T, et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517:50–5. doi: 10.1038/nature14063 - DOI - PMC - PubMed
-
- Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, et al. Structure of a mammalian ryanodine receptor. Nature. 2015;517:44–9. doi: 10.1038/nature13950 - DOI - PMC - PubMed
-
- Clarke NF, Waddell LB, Cooper ST, Perry M, Smith RL, Kornberg AJ, et al. Recessive mutations in RYR1 are a common cause of congenital fiber type disproportion. Hum Mutat. 2010;31:E1544–50. doi: 10.1002/humu.21278 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

