The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease
- PMID: 27911701
- PMCID: PMC5095923
- DOI: 10.1042/BST20160172
The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease
Abstract
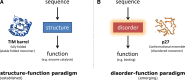
In the 1960s, Christian Anfinsen postulated that the unique three-dimensional structure of a protein is determined by its amino acid sequence. This work laid the foundation for the sequence-structure-function paradigm, which states that the sequence of a protein determines its structure, and structure determines function. However, a class of polypeptide segments called intrinsically disordered regions does not conform to this postulate. In this review, I will first describe established and emerging ideas about how disordered regions contribute to protein function. I will then discuss molecular principles by which regulatory mechanisms, such as alternative splicing and asymmetric localization of transcripts that encode disordered regions, can increase the functional versatility of proteins. Finally, I will discuss how disordered regions contribute to human disease and the emergence of cellular complexity during organismal evolution.
Keywords: RNA localization; alternative splicing; biological networks; gene expression and regulation; intrinsically disordered proteins; protein turnover.
© 2016 The Author(s).
Figures


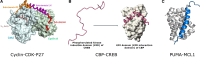





References
-
- Romero P., Obradovic Z., Kissinger C.R., Villafranca J.E., Garner E., Guilliot S. et al. (1998) Thousands of proteins likely to have long disordered regions. Pac. Symp. Biocomput. 437–448 PMID: - PubMed

