WASP family proteins, more than Arp2/3 activators
- PMID: 27911716
- PMCID: PMC5095904
- DOI: 10.1042/BST20160176
WASP family proteins, more than Arp2/3 activators
Abstract
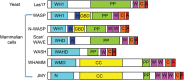
Wiskott-Aldrich syndrome protein (WASP) family proteins have been extensively characterized as factors that promote the nucleation of actin through the activation of the protein complex Arp2/3. While yeast mostly have a single member of the family, mammalian cells have at least six different members, often with multiple isoforms. Members of the family are characterized by a common structure. Their N-termini are varied and are considered to confer spatial and temporal regulation of Arp2/3-activating activity, whereas their C-terminal half contains a polyproline-rich region, one or more WASP homology-2 (WH2) actin-binding domains and motifs that bind directly to Arp2/3. Recent studies, however, indicate that the yeast WASP homologue Las17 is able to nucleate actin independently of Arp2/3 through the function of novel G-actin-binding activities in its polyproline region. This allows Las17 to generate the mother filaments that are needed for subsequent Arp2/3 recruitment and activation during the actin polymerization that drives endocytic invagination in yeast. In this review, we consider how motifs within the polyproline region of Las17 support nucleation of actin filaments, and whether similar mechanisms might exist among other family members.
Keywords: Arp2/3; Las17; WH2; actin; nucleation; polyproline.
© 2016 The Author(s).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

