Multimodal Imaging for DREADD-Expressing Neurons in Living Brain and Their Application to Implantation of iPSC-Derived Neural Progenitors
- PMID: 27911758
- PMCID: PMC6601716
- DOI: 10.1523/JNEUROSCI.1279-16.2016
Multimodal Imaging for DREADD-Expressing Neurons in Living Brain and Their Application to Implantation of iPSC-Derived Neural Progenitors
Abstract
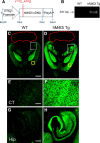
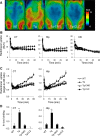
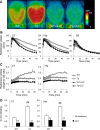
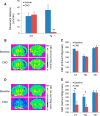
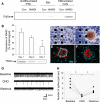
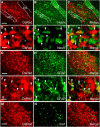
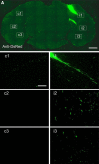
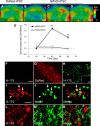
Chemogenetic manipulation of neuronal activities has been enabled by a designer receptor (designer receptor exclusively activated by designer drugs, DREADD) that is activated exclusively by clozapine-N-oxide (CNO). Here, we applied CNO as a functional reporter probe to positron emission tomography (PET) of DREADD in living brains. Mutant human M4 DREADD (hM4Di) expressed in transgenic (Tg) mouse neurons was visualized by PET with microdose [11C]CNO. Deactivation of DREADD-expressing neurons in these mice by nonradioactive CNO at a pharmacological dose could also be captured by arterial spin labeling MRI (ASL-MRI). Neural progenitors derived from hM4Di Tg-induced pluripotent stem cells were then implanted into WT mouse brains and neuronal differentiation of the grafts could be imaged by [11C]CNO-PET. Finally, ASL-MRI captured chemogenetic functional manipulation of the graft neurons. Our data provide the first demonstration of multimodal molecular/functional imaging of cells expressing a functional gene reporter in the brain, which would be translatable to humans for therapeutic gene transfers and cell replacements.
Significance statement: The present work provides the first successful demonstration of in vivo positron emission tomographic (PET) visualization of a chemogenetic designer receptor (designer receptor exclusively activated by designer drugs, DREADD) expressed in living brains. This technology has been applied to longitudinal PET reporter imaging of neuronal grafts differentiated from induced pluripotent stem cells. Differentiated from currently used reporter genes for neuroimaging, DREADD has also been available for functional manipulation of target cells, which could be visualized by functional magnetic resonance imaging (fMRI) in a real-time manner. Multimodal imaging with PET/fMRI enables the visualization of the differentiation of iPSC-derived neural progenitors into mature neurons and DREADD-mediated functional manipulation along the time course of the graft and is accordingly capable of fortifying the utility of stem cells in cell replacement therapies.
Keywords: cell replacement therapy; clozapine-N-oxide (CNO); designer receptor exclusively activated by designer drugs (DREADD); induced pluripotent stem cell (iPSC); positron emission tomography (PET).
Copyright © 2016 the authors 0270-6474/16/3611545-15$15.00/0.
Figures
References
-
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. - DOI - PMC - PubMed
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
