Optical magnetic detection of single-neuron action potentials using quantum defects in diamond
- PMID: 27911765
- PMCID: PMC5150388
- DOI: 10.1073/pnas.1601513113
Optical magnetic detection of single-neuron action potentials using quantum defects in diamond
Erratum in
-
Correction for Barry et al., Optical magnetic detection of single-neuron action potentials using quantum defects in diamond.Proc Natl Acad Sci U S A. 2017 Aug 8;114(32):E6730. doi: 10.1073/pnas.1712523114. Proc Natl Acad Sci U S A. 2017. PMID: 28784779 Free PMC article. No abstract available.
Abstract
Magnetic fields from neuronal action potentials (APs) pass largely unperturbed through biological tissue, allowing magnetic measurements of AP dynamics to be performed extracellularly or even outside intact organisms. To date, however, magnetic techniques for sensing neuronal activity have either operated at the macroscale with coarse spatial and/or temporal resolution-e.g., magnetic resonance imaging methods and magnetoencephalography-or been restricted to biophysics studies of excised neurons probed with cryogenic or bulky detectors that do not provide single-neuron spatial resolution and are not scalable to functional networks or intact organisms. Here, we show that AP magnetic sensing can be realized with both single-neuron sensitivity and intact organism applicability using optically probed nitrogen-vacancy (NV) quantum defects in diamond, operated under ambient conditions and with the NV diamond sensor in close proximity (∼10 µm) to the biological sample. We demonstrate this method for excised single neurons from marine worm and squid, and then exterior to intact, optically opaque marine worms for extended periods and with no observed adverse effect on the animal. NV diamond magnetometry is noninvasive and label-free and does not cause photodamage. The method provides precise measurement of AP waveforms from individual neurons, as well as magnetic field correlates of the AP conduction velocity, and directly determines the AP propagation direction through the inherent sensitivity of NVs to the associated AP magnetic field vector.
Keywords: action potential; magnetometry; neuron; nitrogen-vacancy center.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
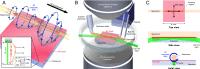

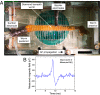

References
-
- Arai K, et al. Fourier magnetic imaging with nanoscale resolution and compressed sensing speed-up using electronic spins in diamond. Nat Nanotechnol. 2015;10(10):859–864. - PubMed
-
- Doherty MW, et al. The nitrogen-vacancy colour centre in diamond. Phys Rep. 2013;528(1):1–45.
-
- Fu RR, et al. Paleomagnetism. Solar nebula magnetic fields recorded in the Semarkona meteorite. Science. 2014;346(6213):1089–1092. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

