Genomic sequencing-based mutational enrichment analysis identifies motility genes in a genetically intractable gut microbe
- PMID: 27911803
- PMCID: PMC5150387
- DOI: 10.1073/pnas.1612753113
Genomic sequencing-based mutational enrichment analysis identifies motility genes in a genetically intractable gut microbe
Abstract
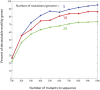
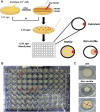
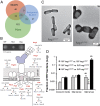
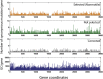
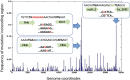
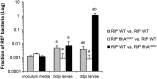
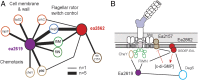
A major roadblock to understanding how microbes in the gastrointestinal tract colonize and influence the physiology of their hosts is our inability to genetically manipulate new bacterial species and experimentally assess the function of their genes. We describe the application of population-based genomic sequencing after chemical mutagenesis to map bacterial genes responsible for motility in Exiguobacterium acetylicum, a representative intestinal Firmicutes bacterium that is intractable to molecular genetic manipulation. We derived strong associations between mutations in 57 E. acetylicum genes and impaired motility. Surprisingly, less than half of these genes were annotated as motility-related based on sequence homologies. We confirmed the genetic link between individual mutations and loss of motility for several of these genes by performing a large-scale analysis of spontaneous suppressor mutations. In the process, we reannotated genes belonging to a broad family of diguanylate cyclases and phosphodiesterases to highlight their specific role in motility and assigned functions to uncharacterized genes. Furthermore, we generated isogenic strains that allowed us to establish that Exiguobacterium motility is important for the colonization of its vertebrate host. These results indicate that genetic dissection of a complex trait, functional annotation of new genes, and the generation of mutant strains to define the role of genes in complex environments can be accomplished in bacteria without the development of species-specific molecular genetic tools.
Keywords: comparative genomics; gene annotation; motility; mutagenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146(6):1525–1533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

