MyTH4-FERM myosins have an ancient and conserved role in filopod formation
- PMID: 27911821
- PMCID: PMC5167205
- DOI: 10.1073/pnas.1615392113
MyTH4-FERM myosins have an ancient and conserved role in filopod formation
Abstract
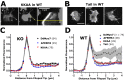
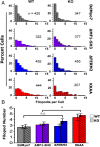
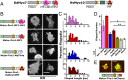
The formation of filopodia in Metazoa and Amoebozoa requires the activity of myosin 10 (Myo10) in mammalian cells and of Dictyostelium unconventional myosin 7 (DdMyo7) in the social amoeba Dictyostelium However, the exact roles of these MyTH4-FERM myosins (myosin tail homology 4-band 4.1, ezrin, radixin, moesin; MF) in the initiation and elongation of filopodia are not well defined and may reflect conserved functions among phylogenetically diverse MF myosins. Phylogenetic analysis of MF myosin domains suggests that a single ancestral MF myosin existed with a structure similar to DdMyo7, which has two MF domains, and that subsequent duplications in the metazoan lineage produced its functional homolog Myo10. The essential functional features of the DdMyo7 myosin were identified using quantitative live-cell imaging to characterize the ability of various mutants to rescue filopod formation in myo7-null cells. The two MF domains were found to function redundantly in filopod formation with the C-terminal FERM domain regulating both the number of filopodia and their elongation velocity. DdMyo7 mutants consisting solely of the motor plus a single MyTH4 domain were found to be capable of rescuing the formation of filopodia, establishing the minimal elements necessary for the function of this myosin. Interestingly, a chimeric myosin with the Myo10 MF domain fused to the DdMyo7 motor also was capable of rescuing filopod formation in the myo7-null mutant, supporting fundamental functional conservation between these two distant myosins. Together, these findings reveal that MF myosins have an ancient and conserved role in filopod formation.
Keywords: MyTH4-FERM; actin; cell motility; filopodia; myosin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Mattila PK, Lappalainen P. Filopodia: Molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9(6):446–454. - PubMed
-
- Tuxworth RI, et al. A role for myosin VII in dynamic cell adhesion. Curr Biol. 2001;11(5):318–329. - PubMed
-
- Ostap EM, et al. Dynamic localization of myosin-I to endocytic structures in Acanthamoeba. Cell Motil Cytoskeleton. 2003;54(1):29–40. - PubMed
-
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22(5):617–625. - PubMed
-
- Mortimer D, Fothergill T, Pujic Z, Richards LJ, Goodhill GJ. Growth cone chemotaxis. Trends Neurosci. 2008;31(2):90–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

