Evaluating the evaluation of cancer driver genes
- PMID: 27911828
- PMCID: PMC5167163
- DOI: 10.1073/pnas.1616440113
Evaluating the evaluation of cancer driver genes
Abstract
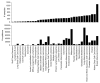
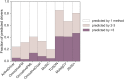
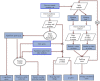
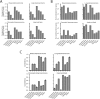
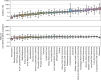
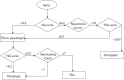
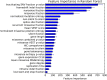
Sequencing has identified millions of somatic mutations in human cancers, but distinguishing cancer driver genes remains a major challenge. Numerous methods have been developed to identify driver genes, but evaluation of the performance of these methods is hindered by the lack of a gold standard, that is, bona fide driver gene mutations. Here, we establish an evaluation framework that can be applied to driver gene prediction methods. We used this framework to compare the performance of eight such methods. One of these methods, described here, incorporated a machine-learning-based ratiometric approach. We show that the driver genes predicted by each of the eight methods vary widely. Moreover, the P values reported by several of the methods were inconsistent with the uniform values expected, thus calling into question the assumptions that were used to generate them. Finally, we evaluated the potential effects of unexplained variability in mutation rates on false-positive driver gene predictions. Our analysis points to the strengths and weaknesses of each of the currently available methods and offers guidance for improving them in the future.
Keywords: DNA sequencing; cancer genomics; cancer mutations; computational method evaluation; driver genes.
Conflict of interest statement
B.V. is a founder of PapGene and Personal Genome Diagnostics and a member of the Scientific Advisory Boards of Morphotek, Syxmex-Inostics, and Exelixis GP. The first four of these companies, as well as other companies, have licensed technologies from Johns Hopkins University, on which B.V. is an inventor. These licenses and relationships are associated with equity or royalty payments to B.V. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. K.W.K. is a founder of PapGene and Personal Genome Diagnostics and a member of the Scientific Advisory Boards of Morphotek and Syxmex-Inostics. These companies, as well as other companies, have licensed technologies from Johns Hopkins University, on which K.W.K. is an inventor. These licenses and relationships are associated with equity or royalty payments to K.W.K. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies. N.P. is a founder of PapGene and Personal Genome Diagnostics. These companies, as well as other companies, have licensed technologies from Johns Hopkins University, on which N.P. is an inventor. These licenses and relationships are associated with equity or royalty payments to N.P. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
Figures





References
-
- Sjöblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

