Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques
- PMID: 27911897
- PMCID: PMC5135040
- DOI: 10.1371/journal.pntd.0005168
Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques
Abstract
Background: Zika virus (ZIKV; Flaviviridae, Flavivirus) was declared a public health emergency of international concern by the World Health Organization (WHO) in February 2016, because of the evidence linking infection with ZIKV to neurological complications, such as Guillain-Barre Syndrome in adults and congenital birth defects including microcephaly in the developing fetus. Because development of a ZIKV vaccine is a top research priority and because the genetic and antigenic variability of many RNA viruses limits the effectiveness of vaccines, assessing whether immunity elicited against one ZIKV strain is sufficient to confer broad protection against all ZIKV strains is critical. Recently, in vitro studies demonstrated that ZIKV likely circulates as a single serotype. Here, we demonstrate that immunity elicited by African lineage ZIKV protects rhesus macaques against subsequent infection with Asian lineage ZIKV.
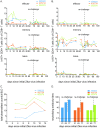
Methodology/principal findings: Using our recently developed rhesus macaque model of ZIKV infection, we report that the prototypical ZIKV strain MR766 productively infects macaques, and that immunity elicited by MR766 protects macaques against heterologous Asian ZIKV. Furthermore, using next generation deep sequencing, we found in vivo restoration of a putative N-linked glycosylation site upon replication in macaques that is absent in numerous MR766 strains that are widely being used by the research community. This reversion highlights the importance of carefully examining the sequence composition of all viral stocks as well as understanding how passage history may alter a virus from its original form.
Conclusions/significance: An effective ZIKV vaccine is needed to prevent infection-associated fetal abnormalities. Macaques whose immune responses were primed by infection with East African ZIKV were completely protected from detectable viremia when subsequently rechallenged with heterologous Asian ZIKV. Therefore, these data suggest that immunogen selection is unlikely to adversely affect the breadth of vaccine protection, i.e., any Asian ZIKV immunogen that protects against homologous challenge will likely confer protection against all other Asian ZIKV strains.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Hayden E (2016) The race is on to develop Zika vaccine. Nature http://www.nature.com/news/the-race-is-on-to-develop-zika-vaccine-1.19634 via the Internet. Accessed x.
-
- McCrae AW, Kirya BG (1982) Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg 76: 552–562. - PubMed
-
- Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ et al. (2001) Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg 64: 310–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

