Characterization of Hippo Pathway Components by Gene Inactivation
- PMID: 27912098
- PMCID: PMC5137798
- DOI: 10.1016/j.molcel.2016.10.034
Characterization of Hippo Pathway Components by Gene Inactivation
Abstract
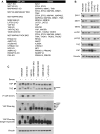
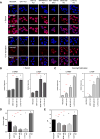
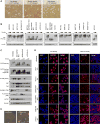
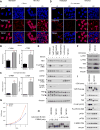
The Hippo pathway is important for regulating tissue homeostasis, and its dysregulation has been implicated in human cancer. However, it is not well understood how the Hippo pathway becomes dysregulated because few mutations in core Hippo pathway components have been identified. Therefore, much work in the Hippo field has focused on identifying upstream regulators, and a complex Hippo interactome has been identified. Nevertheless, it is not always clear which components are the most physiologically relevant in regulating YAP/TAZ. To provide an overview of important Hippo pathway components, we created knockout cell lines for many of these components and compared their relative contributions to YAP/TAZ regulation in response to a wide range of physiological signals. By this approach, we provide an overview of the functional importance of many Hippo pathway components and demonstrate NF2 and RHOA as important regulators of YAP/TAZ and TAOK1/3 as direct kinases for LATS1/2.
Keywords: CRISPR; Hippo pathway; NF2; RHOA; TAOK1; TAOK3; TAZ; YAP.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

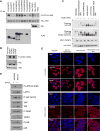
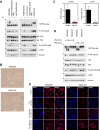
References
-
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. - PubMed
-
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

