Sex and Age Differences in Epinephrine Mechanisms and Outcomes after Brain Injury
- PMID: 27912253
- PMCID: PMC5397223
- DOI: 10.1089/neu.2016.4770
Sex and Age Differences in Epinephrine Mechanisms and Outcomes after Brain Injury
Retraction in
-
Retraction of: Sex and Age Differences in Epinephrine Mechanisms and Outcomes after Brain Injury (DOI: 10.1089/neu.2016.4770).J Neurotrauma. 2022 Jun;39(11-12):894. doi: 10.1089/neu.2016.4770.retract. Epub 2022 Apr 29. J Neurotrauma. 2022. PMID: 35486824 Free PMC article. No abstract available.
Abstract
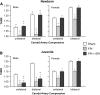
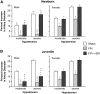
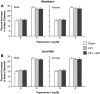
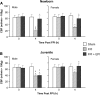
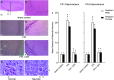
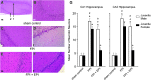
Traumatic brain injury (TBI) is the leading cause of injury-related death in children, with boys and children <4 years of age having particularly poor outcomes. Cerebral autoregulation is often impaired after TBI, contributing to poor outcome. Cerebral perfusion pressure can be normalized by use of vasoactive agents. The c-Jun-terminal kinase (JNK) isoform of mitogen activated protein kinase (MAPK) produces hemodynamic impairment after TBI, but less is known about its role in histopathology. We investigated whether epinephrine (EPI), age, and sex dependently protected cerebral autoregulation and limited histopathology after TBI, and sought to determine the role of JNK in that outcome. Lateral fluid percussion injury (FPI) was produced in anesthetized pigs. Pial artery reactivity was measured via a closed cranial window. Phosphorylated JNK MAPK was quantified by enzyme-linked immunosorbent assay (ELISA). Results show that EPI preserves autoregulation, prevents histopathology, and blocks phosphorylated JNK upregulation in newborn males and females and juvenile females but not juvenile males after TBI. These data indicate that EPI preserves cerebral autoregulation and limits histopathology after TBI through blockade of JNK in an age- and sex-dependent manner.
Keywords: age; brain injury; cerebral autoregulation; histopathology; sex; signal transduction; vasopressor.
Conflict of interest statement
No competing financial interests exist.
Figures

Comment in
-
Findings of Research Misconduct.Fed Regist. 2023 Jul 7;88(129):43363-43364. Fed Regist. 2023. PMID: 37434991 Free PMC article. No abstract available.
References
-
- Langlois J.A., Rutland-Brown W., and Thomas K.E. (2005). The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 20, 229–238 - PubMed
-
- Newacheck P.W., Inkelas M., and Kim S.E. (2004). Heath services use and health care expenditures for children with disabilities. Pediatrics 114, 79–85 - PubMed
-
- Freeman S.S, Udomphorn Y., Armstead W.M., Fisk D.M., and Vavilala M.S. (2008). Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology 108, 588–595 - PubMed
-
- Kochanek P.M., Carney N., Adelson P.D., Aswhal S., Bell M.J., Bratton S., Carson S., Chesnut R.M., Goldstein B., Grant G.A., Kisson N., Peterson K., Selden N.R., Tasker R.C., Tong K.A., Vavilala M.S., Wainwright M.S., and Warden C.R. (2012). Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents–Second Edition. Pediatr. Crit. Care Med. 13, Suppl 1., S24–S29 - PubMed
-
- Ishikawa S., Ito H., Yokoyama K., and Makita K. (2009). Phenylephrine ameliorates cerebral cyotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid occlusion with severe hypotension. Anesth. Analg. 108, 1631–1637 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

