A Novel Rhamnose-Rich Hetero-exopolysaccharide Isolated from Lactobacillus paracasei DG Activates THP-1 Human Monocytic Cells
- PMID: 27913418
- PMCID: PMC5244303
- DOI: 10.1128/AEM.02702-16
A Novel Rhamnose-Rich Hetero-exopolysaccharide Isolated from Lactobacillus paracasei DG Activates THP-1 Human Monocytic Cells
Abstract
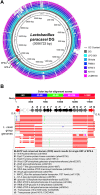
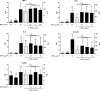
Lactobacillus paracasei DG is a bacterial strain with recognized probiotic properties and is used in commercial probiotic products. However, the mechanisms underlying its probiotic properties are mainly unknown. In this study, we tested the hypothesis that the ability of strain DG to interact with the host is at least partly associated with its ability to synthesize a surface-associated exopolysaccharide (EPS). Comparative genomics revealed the presence of putative EPS gene clusters in the DG genome; accordingly, EPS was isolated from the surface of the bacterium. A sample of the pure EPS from strain DG (DG-EPS), upon nuclear magnetic resonance (NMR) and chemical analyses, was shown to be a novel branched hetero-EPS with a repeat unit composed of l-rhamnose, d-galactose, and N-acetyl-d-galactosamine in a ratio of 4:1:1. Subsequently, we demonstrated that DG-EPS displays immunostimulating properties by enhancing the gene expression of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), and particularly that of the chemokines IL-8 and CCL20, in the human monocytic cell line THP-1. In contrast, the expression of the cyclooxygenase enzyme COX-2 was not affected. In conclusion, DG-EPS is a bacterial macromolecule with the ability to boost the immune system either as a secreted molecule released from the bacterium or as a capsular envelope on the bacterial cell wall. This study provides additional information about the mechanisms supporting the cross talk between L. paracasei DG and the host.
Importance: The consumption of food products and supplements called probiotics (i.e., containing live microbial cells) to potentially prevent or treat specific diseases is constantly gaining popularity. The lack of knowledge on the precise mechanisms supporting their potential health-promoting properties, however, greatly limits a more appropriate use of each single probiotic strain. In this context, we studied a well-known probiotic, Lactobacillus paracasei DG, in order to identify the constitutive molecules that can explain the documented health-promoting properties of this bacterium. We found a novel polysaccharide molecule, named DG-EPS, that is secreted by and covers the bacterium. We demonstrated that this molecule, which has a chemical structure never identified before, has immunostimulatory properties and therefore may contribute to the ability of the probiotic L. paracasei DG to interact with the immune system.
Keywords: Capsular EPS; Enterolactis; Lactobacillus casei DG; Lactobacillus paracasei; MAMPs; THP-1; immunostimulation; probiotic.
Copyright © 2017 American Society for Microbiology.
Figures


References
-
- D'Inca R, Barollo M, Scarpa M, Grillo AR, Brun P, Vettorato MG, Castagliuolo I, Sturniolo GC. 2011. Rectal administration of Lactobacillus casei DG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis. Dig Dis Sci 56:1178–1187. doi:10.1007/s10620-010-1384-1. - DOI - PubMed
-
- Tursi A, Brandimarte G, Giorgetti GM, Modeo ME. 2004. Effect of Lactobacillus casei supplementation on the effectiveness and tolerability of a new second-line 10-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med Sci Monit 10:CR662–CR666. - PubMed
-
- Rosania R, Giorgio F, Principi M, Amoruso A, Monno R, Di Leo A, Ierardi E. 2013. Effect of probiotic or prebiotic supplementation on antibiotic therapy in the small intestinal bacterial overgrowth: a comparative evaluation. Curr Clin Pharmacol 8:169–172. doi:10.2174/15748847113089990048. - DOI - PubMed
-
- Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, Stuknyte M, Chouaia B, Riso P, Guglielmetti S. 2014. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr 144:1787–1796. doi:10.3945/jn.114.197723. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

