A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease
- PMID: 27913695
- PMCID: PMC5224719
- DOI: 10.1212/WNL.0000000000003478
A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease
Abstract
Objective: To test the hypothesis that chronic treatment of early-stage Huntington disease (HD) with high-dose coenzyme Q10 (CoQ) will slow the progressive functional decline of HD.
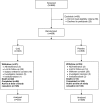
Methods: We performed a multicenter randomized, double-blind, placebo-controlled trial. Patients with early-stage HD (n = 609) were enrolled at 48 sites in the United States, Canada, and Australia from 2008 to 2012. Patients were randomized to receive either CoQ 2,400 mg/d or matching placebo, then followed for 60 months. The primary outcome variable was the change from baseline to month 60 in Total Functional Capacity score (for patients who survived) combined with time to death (for patients who died) analyzed using a joint-rank analysis approach.
Results: An interim analysis for futility revealed a conditional power of <5% for the primary analysis, prompting premature conclusion in July 2014. No statistically significant differences were seen between treatment groups for the primary or secondary outcome measures. CoQ was generally safe and well-tolerated throughout the study.
Conclusions: These data do not justify use of CoQ as a treatment to slow functional decline in HD.
Clinicaltrialsgov identifier: NCT00608881.
Classification of evidence: This article provides Class I evidence that CoQ does not slow the progressive functional decline of patients with HD.
© 2016 American Academy of Neurology.
Figures
Comment in
-
Is mitochondrial oxidative metabolism the right therapy target in early Huntington disease?Neurology. 2017 Jan 10;88(2):116-117. doi: 10.1212/WNL.0000000000003497. Epub 2016 Dec 2. Neurology. 2017. PMID: 27913697 No abstract available.
References
-
- Ross CA, Aylward EH, Wild EJ, et al. . Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 2014;10:204–216. - PubMed
-
- Rüb U, Vonsettel JP, Heinsen H, Korf HW. The neuropathology of Huntington's disease: classical findings, recent developments and correlation to functional neuroanatomy. Adv Anat Embryol Cell Biol 2015;217:1–146. - PubMed
-
- Dayalu P, Albin RL. Huntington disease: pathogenesis and treatment. Neurol Clin 2015;33:101–114. - PubMed
-
- Sampaio C, Borowsky B, Reilmann R. Clinical trials in Huntington's disease: interventions in early clinical development and newer methodological approaches. Mov Disord 2014;29:1419–1428. - PubMed
-
- Zielonka D, Mielcarek M, Landwehrmeyer GB. Update on Huntington's disease: advances in care and emerging therapeutic options. Parkinsonism Relat Disord 2015;21:169–178. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials