The role of macromolecules in the formation of kidney stones
- PMID: 27913854
- PMCID: PMC5253101
- DOI: 10.1007/s00240-016-0948-8
The role of macromolecules in the formation of kidney stones
Abstract
The formation of crystal aggregates, one of the critical processes in kidney stone pathogenesis, involves interactions between crystals (predominantly calcium oxalate monohydrate, COM) and urinary constituents (e.g., proteins), which serve as an adhesive "glue" between crystals in stones. To develop a better understanding of the protein-crystal interactions that lead to crystal aggregation, we have measured the effect of model proteins on bulk COM crystal properties as well as their adsorption on crystal surfaces using three synthetic polyanions: poly(aspartic acid) (polyD), poly(glutamic acid) (polyE), and poly(acrylic acid) (polyAA). These anionic macromolecules reduced the amount of COM crystal aggregation in bulk solution to an extent similar to that observed for mixture of proteins from normal urine, with little difference between the polymers. In contrast, the polymers exhibited differences in measures of COM crystal growth. Polycations such as poly(arginine) (polyR) and poly(lysine) (polyK) reduced aggregation weakly and exerted negligible effects on crystal growth. All polyions were found to associate with COM crystal surfaces, as evidenced by changes in the zeta potential of COM crystals in electrophoretic mobility measurements. On the other hand, COM aggregation and possibly growth can be promoted by many binary mixtures of polycations and polyanions, which appeared to be mediated by polymer aggregate formation rather than loss of crystal charge stabilization. Similarly, crystal aggregation promotion behavior can be driven by forming aggregates of weakly charged polyanions, like Tamm-Horsfall protein, suggesting that polymer (protein) aggregation may play a critical role in stone formation. Sensitivity of polyanion-COM crystal surface interactions to the chemical composition of polymer side groups were demonstrated by large differences in crystal aggregation behavior between polyD and polyE, which correlated with atomic force microscopy (AFM) measurements of growth inhibition on various COM surfaces and chemical force microscopy (CFM) measurements of unbinding forces between COM crystal surfaces and AFM tips decorated with either carboxylate or amidinium moieties (mimicking polyanion and polyR side chains, respectively). The lack of strong interaction for polyE at the COM (100) surface compared to polyD appeared to be the critical difference. Finally, the simultaneous presence of polyanions and polycations appeared to alter the ability of polycations to mediate unbinding forces in CFM and promote crystal growth. In summary, polyanions strongly associated with COM surfaces and influenced crystallization, while polycations did not, though important differences were observed based on the physicochemical properties of polyanions. Observations suggest that COM aggregation with both polyanion-polycation mixtures and weakly charged polyanions is promoted by polymer aggregate formation, which plays a critical role in bridging crystal surfaces.
Keywords: Adhesion; Aggregation; Atomic force microscopy; Calcium oxalate; Kidney stone; Polyelectrolyte.
Conflict of interest statement
Ethical approval: None of the authors has any conflicts of interest to report.
Figures


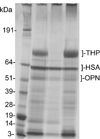

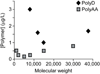


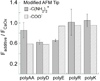




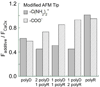
References
-
- Kaiser ET, Bock SC. Protein inhibitors of crystal-growth. J Urology. 1989;141(3):750–752. - PubMed
-
- Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hy. 2006;15(4):374–380. - PubMed
-
- Ryall RL. Glycosaminoglycans, proteins, and stone formation: Adult themes and child's play. Pediatr Nephrol. 1996;10(5):656–666. - PubMed
-
- Boyce WH, Garvey FK. The amount and nature of the organic matrix in urinary calculi - a review. J Urology. 1956;76(3):213–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

