Antifreeze glycopeptides: from structure and activity studies to current approaches in chemical synthesis
- PMID: 27913993
- PMCID: PMC5274654
- DOI: 10.1007/s00726-016-2368-z
Antifreeze glycopeptides: from structure and activity studies to current approaches in chemical synthesis
Erratum in
-
Erratum to: Antifreeze glycopeptides: from structure and activity studies to current approaches in chemical synthesis.Amino Acids. 2017 Apr;49(4):805. doi: 10.1007/s00726-017-2381-x. Amino Acids. 2017. PMID: 28132090 Free PMC article. No abstract available.
Abstract
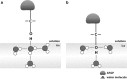
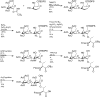
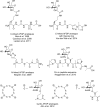
Antifreeze glycopeptides (AFGPs) are a class of biological antifreeze agents found predominantly in Arctic and Antarctic species of fish. They possess the ability to regulate ice nucleation and ice crystal growth, thus creating viable life conditions at temperatures below the freezing point of body fluids. AFGPs usually consist of 4-55 repetitions of the tripeptide unit Ala-Ala-Thr that is O-glycosylated at the threonine side chains with β-D-galactosyl-(1 → 3)-α-N-acetyl-D-galactosamine. Due to their interesting properties and high antifreeze activity, they have many potential applications, e.g., in food industry and medicine. Current research is focused towards understanding the relationship between the structural preferences and the activity of the AFGPs, as well as developing time and cost efficient ways of synthesis of this class of molecules. Recent computational studies in conjunction with experimental results from NMR and THz spectroscopies were a possible breakthrough in understanding the mechanism of action of AFGPs. At the moment, as a result of these findings, the focus of research is shifted towards the analysis of behaviour of the hydration shell around AFGPs and the impact of water-dynamics retardation caused by AFGPs on ice crystal growth. In the field of organic synthesis of AFGP analogues, most of the novel protocols are centered around solid-phase peptide synthesis and multiple efforts are made to optimize this approach. In this review, we present the current state of knowledge regarding the structure and activity of AFGPs, as well as approaches to organic synthesis of these molecules with focus on the most recent developments.
Keywords: AFGP; Antifreeze glycopeptides; Hydration shell dynamics; Solid-phase peptide synthesis; Structure–activity relationship; Terahertz spectroscopy.
Conflict of interest statement
The authors declare that they have no conflict of interest. Ethical approval The article does not contain or refer to any studies with human participants or animals performed by any of the authors.
Figures




References
-
- Ahn M, Murugan RN, Shin SY, Kim E, Lee JH, Kim HJ, Bang JK. Synthesis of cyclic antifreeze glycopeptide and glycopeptoids and their ice recrystallization inhibition activity. Bull Korean Chem Soc. 2012;33:3565–3570. doi: 10.5012/bkcs.2012.33.11.3565. - DOI
-
- Ahn M, Murugan RN, Shin SY, Kim HJ, Bang JK. Peptoid-based positional scanning derivatives: revealing the optimum residue required for ice recrystallization inhibition activity for every position in the AFGPs. Bull Korean Chem Soc. 2012;33:3931–3932. doi: 10.5012/bkcs.2012.33.12.3931. - DOI
-
- Ananthanarayanan VS. Antifreeze proteins: structural diversity and mechanism of action. Life Chem Rep. 1989;7:1–32.
-
- Balcerzak AK, Capicciotti CJ, Briard JG, Ben RN. Designing ice recrystallization inhibitors: from antifreeze (glyco)proteins to small molecules. RCS Adv. 2014;4:42682–42696.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

