Assembling the Tat protein translocase
- PMID: 27914200
- PMCID: PMC5201420
- DOI: 10.7554/eLife.20718
Assembling the Tat protein translocase
Abstract
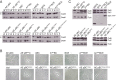
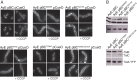
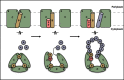
The twin-arginine protein translocation system (Tat) transports folded proteins across the bacterial cytoplasmic membrane and the thylakoid membranes of plant chloroplasts. The Tat transporter is assembled from multiple copies of the membrane proteins TatA, TatB, and TatC. We combine sequence co-evolution analysis, molecular simulations, and experimentation to define the interactions between the Tat proteins of Escherichia coli at molecular-level resolution. In the TatBC receptor complex the transmembrane helix of each TatB molecule is sandwiched between two TatC molecules, with one of the inter-subunit interfaces incorporating a functionally important cluster of interacting polar residues. Unexpectedly, we find that TatA also associates with TatC at the polar cluster site. Our data provide a structural model for assembly of the active Tat translocase in which substrate binding triggers replacement of TatB by TatA at the polar cluster site. Our work demonstrates the power of co-evolution analysis to predict protein interfaces in multi-subunit complexes.
Keywords: E. coli; Tat protein transport; biochemistry; biophysics; membrane protein; sequence co-evolution; structural biology; twin-arginine.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures













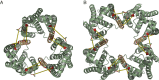

References
MeSH terms
Substances
Grants and funding
- 110183/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- G1001640 /MRC_/Medical Research Council/United Kingdom
- BB/H018050/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L002531/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 107929/Z/15/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

