Renal xenotransplantation: experimental progress and clinical prospects
- PMID: 27914702
- PMCID: PMC5357451
- DOI: 10.1016/j.kint.2016.08.035
Renal xenotransplantation: experimental progress and clinical prospects
Abstract
There are >100,000 patients waiting for kidney transplants in the United States and a vast need worldwide. Xenotransplantation, in the form of the transplantation of kidneys from genetically engineered pigs, offers the possibility of overcoming the chronic shortage of deceased and living human donors. These genetic manipulations can take the form of (i) knockout of pig genes that are responsible for the expression of antigens against which the primate (human or nonhuman primate) has natural "preformed" antibodies that bind and initiate complement-mediated destruction or (ii) the insertion of human transgenes that provide protection against the human complement, coagulation, or inflammatory responses. Between 1989 and 2015, pig kidney graft survival in nonhuman primates increased from 23 days to almost 10 months. There appear to be no clinically significant physiological incompatibilities in renal function between pigs and primates. The organ-source pigs will be housed in a biosecure environment, and thus the risk of transferring an exogenous potentially pathogenic microorganism will be less than that after allotransplantation. Although the risk associated with porcine endogenous retroviruses is considered small, techniques are now available whereby they could potentially be excluded from the pig. The US Food and Drug Administration suggests that xenotransplantation should be restricted to "patients with serious or life-threatening diseases for whom adequately safe and effective alternative therapies are not available." These might include those with (i) a high degree of allosensitization to human leukocyte antigens or (ii) rapid recurrence of primary disease in previous allografts. The potential psychosocial, regulatory, and legal aspects of clinical xenotransplantation are briefly discussed.
Keywords: allosensitization; clinical trial; genetically engineered xenotransplantation; kidney; pig.
Copyright © 2016 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No author reports a conflict of interest.
Figures

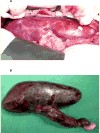

References
-
- Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. 2005;16:1859–1865. - PubMed
-
- Smith JM, Schnitzler MA, Gustafson SK, et al. Cost Implications of New National Allocation Policy for Deceased Donor Kidneys in the United States. Transplantation. 2016;100:879–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

