Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms
- PMID: 27914963
- PMCID: PMC5183552
- DOI: 10.1016/j.neuropharm.2016.11.026
Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms
Abstract
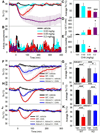
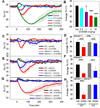
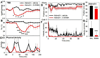
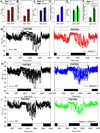
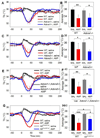
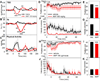
Small mammals have the ability to enter torpor, a hypothermic, hypometabolic state, allowing impressive energy conservation. Administration of adenosine or adenosine 5'-monophosphate (AMP) can trigger a hypothermic, torpor-like state. We investigated the mechanisms for hypothermia using telemetric monitoring of body temperature in wild type and receptor knock out (Adora1-/-, Adora3-/-) mice. Confirming prior data, stimulation of the A3 adenosine receptor (AR) induced hypothermia via peripheral mast cell degranulation, histamine release, and activation of central histamine H1 receptors. In contrast, A1AR agonists and AMP both acted centrally to cause hypothermia. Commonly used, selective A1AR agonists, including N6-cyclopentyladenosine (CPA), N6-cyclohexyladenosine (CHA), and MRS5474, caused hypothermia via both A1AR and A3AR when given intraperitoneally. Intracerebroventricular dosing, low peripheral doses of Cl-ENBA [(±)-5'-chloro-5'-deoxy-N6-endo-norbornyladenosine], or using Adora3-/- mice allowed selective stimulation of A1AR. AMP-stimulated hypothermia can occur independently of A1AR, A3AR, and mast cells. A1AR and A3AR agonists and AMP cause regulated hypothermia that was characterized by a drop in total energy expenditure, physical inactivity, and preference for cooler environmental temperatures, indicating a reduced body temperature set point. Neither A1AR nor A3AR was required for fasting-induced torpor. A1AR and A3AR agonists and AMP trigger regulated hypothermia via three distinct mechanisms.
Keywords: (1R,2R,3S,5S)-4-(2-chloro-6-((dicyclopropylmethyl)amino)-9H-purin-9-yl)bicyclo[3.1.0]hexane-2,3-diol; (±)-5'-chloro-5'-deoxy-N(6)-endo-norbornyladenosine; 2-Chloro-N(6)-cyclopentyladenosine; A(1)AR; A(3)AR; AMP; Adenosine; Adenosine 5'-monophosphate; CCPA; CHA; CPA; Cl-ENBA; Hypothermia; MRS5474; N(6)-(p-sulfo-phenyl)adenosine; N(6)-R-phenylisopropyladenosine; N(6)-cyclohexyladenosine; N(6)-cyclopentyladenosine; R-PIA; SPA; Torpor.
Published by Elsevier Ltd.
Figures



Comment in
-
Therapeutic hypothermia: Turning humans into cold-blooded ectotherms via adenosine receptors.Neuropharmacology. 2017 Apr;116:441-443. doi: 10.1016/j.neuropharm.2016.12.027. Epub 2017 Jan 13. Neuropharmacology. 2017. PMID: 28089845 No abstract available.
Similar articles
-
Peripheral Adenosine A3 Receptor Activation Causes Regulated Hypothermia in Mice That Is Dependent on Central Histamine H1 Receptors.J Pharmacol Exp Ther. 2016 Feb;356(2):474-82. doi: 10.1124/jpet.115.229872. Epub 2015 Nov 25. J Pharmacol Exp Ther. 2016. PMID: 26606937 Free PMC article.
-
Activation of neuronal adenosine A1 receptors causes hypothermia through central and peripheral mechanisms.PLoS One. 2020 Dec 16;15(12):e0243986. doi: 10.1371/journal.pone.0243986. eCollection 2020. PLoS One. 2020. PMID: 33326493 Free PMC article.
-
Central activation of the A1 adenosine receptor in fed mice recapitulates only some of the attributes of daily torpor.J Comp Physiol B. 2017 Jul;187(5-6):835-845. doi: 10.1007/s00360-017-1084-7. Epub 2017 Apr 4. J Comp Physiol B. 2017. PMID: 28378088 Free PMC article.
-
The role of the adenosinergic system in lung fibrosis.Pharmacol Res. 2013 Oct;76:182-9. doi: 10.1016/j.phrs.2013.08.004. Epub 2013 Aug 28. Pharmacol Res. 2013. PMID: 23994158 Review.
-
Partial agonists for A(3) adenosine receptors.Curr Top Med Chem. 2004;4(8):855-62. doi: 10.2174/1568026043450989. Curr Top Med Chem. 2004. PMID: 15078216 Free PMC article. Review.
Cited by
-
Tremorolytic effect of 5'-chloro-5'-deoxy-(±)-ENBA, a potent and selective adenosine A1 receptor agonist, evaluated in the harmaline-induced model in rats.CNS Neurosci Ther. 2017 May;23(5):438-446. doi: 10.1111/cns.12692. Epub 2017 Mar 29. CNS Neurosci Ther. 2017. PMID: 28371468 Free PMC article.
-
Design and in Vivo Characterization of A1 Adenosine Receptor Agonists in the Native Ribose and Conformationally Constrained (N)-Methanocarba Series.J Med Chem. 2019 Feb 14;62(3):1502-1522. doi: 10.1021/acs.jmedchem.8b01662. Epub 2019 Jan 3. J Med Chem. 2019. PMID: 30605331 Free PMC article.
-
The Inside Story of Adenosine.Int J Mol Sci. 2018 Mar 9;19(3):784. doi: 10.3390/ijms19030784. Int J Mol Sci. 2018. PMID: 29522447 Free PMC article. Review.
-
In vivo phenotypic validation of adenosine receptor-dependent activity of non-adenosine drugs.Purinergic Signal. 2023 Sep;19(3):551-564. doi: 10.1007/s11302-023-09924-3. Epub 2023 Feb 13. Purinergic Signal. 2023. PMID: 36781825 Free PMC article.
-
Synthetic torpor protects rats from exposure to accelerated heavy ions.Sci Rep. 2022 Sep 30;12(1):16405. doi: 10.1038/s41598-022-20382-6. Sci Rep. 2022. PMID: 36180516 Free PMC article.
References
-
- Akula KK, Kulkarni SK. Effect of curcumin against pentylenetetrazol-induced seizure threshold in mice: possible involvement of adenosine A1 receptors. Phytother Res. 2014;28(5):714–721. - PubMed
-
- Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database of Systematic Reviews. 2012;9 Art. No.: CD004128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

