Tonotopic representation of loudness in the human cortex
- PMID: 27915027
- PMCID: PMC5256480
- DOI: 10.1016/j.heares.2016.11.015
Tonotopic representation of loudness in the human cortex
Abstract
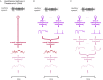
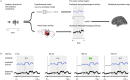
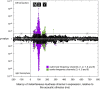
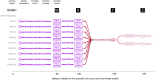
A prominent feature of the auditory system is that neurons show tuning to audio frequency; each neuron has a characteristic frequency (CF) to which it is most sensitive. Furthermore, there is an orderly mapping of CF to position, which is called tonotopic organization and which is observed at many levels of the auditory system. In a previous study (Thwaites et al., 2016) we examined cortical entrainment to two auditory transforms predicted by a model of loudness, instantaneous loudness and short-term loudness, using speech as the input signal. The model is based on the assumption that neural activity is combined across CFs (i.e. across frequency channels) before the transform to short-term loudness. However, it is also possible that short-term loudness is determined on a channel-specific basis. Here we tested these possibilities by assessing neural entrainment to the overall and channel-specific instantaneous loudness and the overall and channel-specific short-term loudness. The results showed entrainment to channel-specific instantaneous loudness at latencies of 45 and 100 ms (bilaterally, in and around Heschl's gyrus). There was entrainment to overall instantaneous loudness at 165 ms in dorso-lateral sulcus (DLS). Entrainment to overall short-term loudness occurred primarily at 275 ms, bilaterally in DLS and superior temporal sulcus. There was only weak evidence for entrainment to channel-specific short-term loudness.
Keywords: Entrainment; Loudness; Magnetoencephalography; Model expression; Temporal integration; Tonotopy.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Brainard D.H. The psychophysics Toolbox. Spat. Vis. 1997;10:433–436. - PubMed
-
- Chalupper J., Fastl H. Dynamic loudness model (DLM) for normal and hearing impaired listeners. Acta Acust. United Acust. 2002;88:378–386.
-
- Crouzet O., Ainsworth W.A. Workshop on Consistent and Reliable Cues for Sound Analysis (pp. 1–4). Aalborg, Denmark. 2001. On the various instances of envelope information on the perception of speech in adverse conditions: an analysis of between-channel envelope correlation.
-
- Davis M., Sigal R., Weyuker E.J. Morgan Kaufmann; Burlington, MA: 1994. Computability, Complexity, and Languages: Fundamentals of Theoretical Computer Science.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

