Resistance to Cell Death and Its Modulation in Cancer Stem Cells
- PMID: 27915972
- PMCID: PMC5356509
- DOI: 10.1615/CritRevOncog.2016016976
Resistance to Cell Death and Its Modulation in Cancer Stem Cells
Abstract
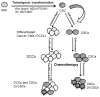
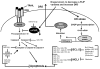
Accumulating evidence has demonstrated that human cancers arise from various tissues of origin that initiate from cancer stem cells (CSCs) or cancer-initiating cells. The extrinsic and intrinsic apoptotic pathways are dysregulated in CSCs, and these cells play crucial roles in tumor initiation, progression, cell death resistance, chemo- and radiotherapy resistance, and tumor recurrence. Understanding CSC-specific signaling proteins and pathways is necessary to identify specific therapeutic targets that may lead to the development of more efficient therapies selectively targeting CSCs. Several signaling pathways-including the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR), maternal embryonic leucine zipper kinase (MELK), NOTCH1, and Wnt/Β-catenin&and expression of the CSC markers CD133, CD24, CD44, Oct4, Sox2, Nanog, and ALDH1A1 maintain CSC properties. Studying such pathways may help to understand CSC biology and lead to the development of potential therapeutic interventions to render CSCs more sensitive to cell death triggered by chemotherapy and radiation therapy. Moreover, recent demonstrations of dedifferentiation of differentiated cancer cells into CSC-like cells have created significant complexity in the CSCs hypothesis. Therefore, any successful therapeutic agent or combination of drugs for cancer therapy must eliminate not only CSCs but differentiated cancer cells and the entire bulk of tumor cells. This review article expands on the CSC hypothesis and paradigm with respect to major signaling pathways and effectors that regulate CSC apoptosis resistance. Moreover, selective CSC apoptotic modulators and their therapeutic potential for making tumors more responsive to therapy are discussed. The use of novel therapies, including small-molecule inhibitors of specific proteins in signaling pathways that regulate stemness, proliferation and migration of CSCs, immunotherapy, and noncoding microRNAs may provide better means of treating CSCs.
Figures

References
-
- Ween MP, Armstrong MA, Oehler MK, Ricciardelli C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol. 2015;96(2):220–56. - PubMed
-
- Roberti A, La Sala D, Cinti C. Multiple genetic and epigenetic interacting mechanisms contribute to clonally selection of drug-resistant tumors: current views and new therapeutic prospective. J Cell Physiol. 2006;207(3):571–81. - PubMed
-
- Wang F, Lin J, Xu R. The molecular mechanisms of TRAIL resistance in cancer cells: help in designing new drugs. Curr Pharm Des. 2014;20(42):6714–22. - PubMed
-
- Tang KD, Ling MT. Targeting drug-resistant prostate cancer with dual PI3K/mTOR inhibition. Curr Med Chem. 2014;21(26):3048–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

