Cyclophilin D over-expression increases mitochondrial complex III activity and accelerates supercomplex formation
- PMID: 27916505
- PMCID: PMC5217830
- DOI: 10.1016/j.abb.2016.11.008
Cyclophilin D over-expression increases mitochondrial complex III activity and accelerates supercomplex formation
Abstract
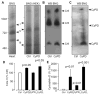
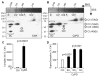
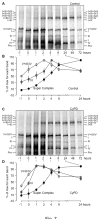
Cyclophilin D (CyPD), a mitochondrial matrix protein, has been widely studied for its role in mitochondrial-mediated cell death. Unexpectedly, we previously discovered that overexpression of CyPD in a stable cell line, increased mitochondrial membrane potentials and enhanced cell survival under conditions of oxidative stress. Here, we investigated the underlying mechanisms responsible for these findings. Spectrophotometric measurements in isolated mitochondria revealed that overexpression of CyPD in HEK293 cells increased respiratory chain activity, but only for Complex III (CIII). Acute treatment of mitochondria with the immumosupressant cyclosporine A did not affect CIII activity. Expression levels of the CIII subunits cytochrome b and Rieske-FeS were elevated in HEK293 cells overexpressing CyPD. However, CIII activity was still significantly higher compared to control mitochondria, even when normalized by protein expression. Blue native gel electrophoresis and Western blot assays revealed a molecular interaction of CyPD with CIII and increased levels of supercomplexes in mitochondrial protein extracts. Radiolabeled protein synthesis in mitochondria showed that CIII assembly and formation of supercomplexes containing CIII were significantly faster when CyPD was overexpressed. Taken together, these data indicate that CyPD regulates mitochondrial metabolism, and likely cell survival, by promoting more efficient electrons flow through the respiratory chain via increased supercomplex formation.
Keywords: Chaperone; Metabolic regulation; Mitochondrial permeability transition (MPT); Mitochondrial respiratory chain complex; Prolyl isomerase.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


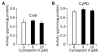

References
-
- Hansford RG. Physiological role of mitochondrial Ca2+ transport. J Bioenerg Biomembr. 1994;26(5):495–508. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

