Expanded Access of Investigational Drugs: The Experience of the Center of Drug Evaluation and Research Over a 10-Year Period
- PMID: 27917324
- PMCID: PMC5135086
- DOI: 10.1177/2168479016656030
Expanded Access of Investigational Drugs: The Experience of the Center of Drug Evaluation and Research Over a 10-Year Period
Abstract
Background: The purpose of this study was to describe the experience of the Center of Drug Evaluation and Research (CDER) with expanded access of investigational drugs.
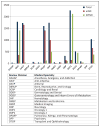
Methods: Multiple searches of CDER's document tracking system were performed to identify the number, type, and indication for all expanded access requests over the 10-year time period of January 2005 through December 2014. An additional search was performed to identify all active commercial investigational drug development programs during that time period and whether or not the clinical program was placed on hold. The two searches were then cross-referenced to identify those commercial investigational drug development programs placed on clinical hold due to serious adverse events occurring within expanded access programs.
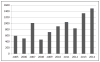
Results: CDER receives over 1000 applications for expanded access each year. The majority are for single patients, roughly evenly split between emergency and nonemergency use. The vast majority, 99.7%, are allowed to proceed. The incidence of clinical holds for all commercial investigational drug development programs is 7.9%, as compared to only 0.2% related to adverse events observed in patients receiving drug treatments under expanded access.
Conclusions: The expanded access program is viewed as a success from FDA's perspective based on the large number of applications processed and allowed to proceed each year. However, the actual number of patients and their health care providers that desire drug treatments available under expanded access is not known. It is exceedingly rare for a serious adverse event under expanded access to affect the development program for that drug.
Keywords: US Food and Drug Administration; compassionate use; expanded access.
Conflict of interest statement
Declaration of Conflicting Interests No potential conflicts were declared.
Figures
References
-
- Rosenblatt M, Kuhlik B. Principles and challenges in access to experimental medicines. JAMA. 2015;313:2023–2024. - PubMed
-
- Caplan A. Medical ethicist Arthur Caplan explains why he opposes “right-to-try” laws. Oncology (Williston Park) 2016;30:8. - PubMed
-
- Caplan AL, Bateman-House A. Should patients in need be given access to experimental drugs? Expert Opin Pharmacother. 2015;16:1275–1279. - PubMed
-
- Bateman-House A, Kimberly L, Redman B, Dubler N, Caplan A. Right-to-try laws: hope, hype, and unintended consequences. Ann Intern Med. 2015;163:796–797. - PubMed
-
- Tsakopoulos A, Han J, Nodler H, Russo V. The right to try: an overview of efforts to obtain expedited access to unapproved treatment for the terminally ill. Food Drug Law J. 2015;70:617–641. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

