Structural analysis of the bright monomeric yellow-green fluorescent protein mNeonGreen obtained by directed evolution
- PMID: 27917830
- PMCID: PMC5137226
- DOI: 10.1107/S2059798316018623
Structural analysis of the bright monomeric yellow-green fluorescent protein mNeonGreen obtained by directed evolution
Abstract
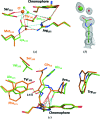
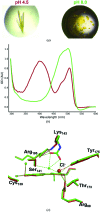
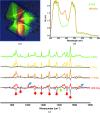
Until recently, genes coding for homologues of the autofluorescent protein GFP had only been identified in marine organisms from the phyla Cnidaria and Arthropoda. New fluorescent-protein genes have now been found in the phylum Chordata, coding for particularly bright oligomeric fluorescent proteins such as the tetrameric yellow fluorescent protein lanYFP from Branchiostoma lanceolatum. A successful monomerization attempt led to the development of the bright yellow-green fluorescent protein mNeonGreen. The structures of lanYFP and mNeonGreen have been determined and compared in order to rationalize the directed evolution process leading from a bright, tetrameric to a still bright, monomeric fluorescent protein. An unusual discolouration of crystals of mNeonGreen was observed after X-ray data collection, which was investigated using a combination of X-ray crystallography and UV-visible absorption and Raman spectroscopies, revealing the effects of specific radiation damage in the chromophore cavity. It is shown that X-rays rapidly lead to the protonation of the phenolate O atom of the chromophore and to the loss of its planarity at the methylene bridge.
Keywords: Branchiostoma lanceolatum; fluorescent protein; in crystallo optical spectroscopy; online Raman spectroscopy; specific radiation damage.
Figures



References
-
- Adam, V., Carpentier, P., Violot, S., Lelimousin, M., Darnault, C., Nienhaus, G. U. & Bourgeois, D. (2009). J. Am. Chem. Soc. 131, 18063–18065. - PubMed
-
- Bell, A. F., He, X., Wachter, R. M. & Tonge, P. J. (2000). Biochemistry, 39, 4423–4431. - PubMed
-
- Burmeister, W. P. (2000). Acta Cryst. D56, 328–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

