High-Frequency Stimulation of the Subthalamic Nucleus Blocks Compulsive-Like Re-Escalation of Heroin Taking in Rats
- PMID: 27917870
- PMCID: PMC5520777
- DOI: 10.1038/npp.2016.270
High-Frequency Stimulation of the Subthalamic Nucleus Blocks Compulsive-Like Re-Escalation of Heroin Taking in Rats
Abstract
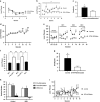
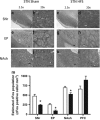
Opioid addiction, including addiction to heroin, has markedly increased in the past decade. The cost and pervasiveness of heroin addiction, including resistance to recovery from addiction, provide a compelling basis for developing novel therapeutic strategies. Deep brain stimulation may represent a viable alternative strategy for the treatment of intractable heroin addiction, particularly in individuals who are resistant to traditional therapies. Here we provide preclinical evidence of the therapeutic potential of high-frequency stimulation of the subthalamic nucleus (STN HFS) for heroin addiction. STN HFS prevented the re-escalation of heroin intake after abstinence in rats with extended access to heroin, an animal model of compulsive heroin taking. STN HFS inhibited key brain regions, including the substantia nigra, entopeduncular nucleus, and nucleus accumbens shell measured using brain mapping analyses of immediate-early gene expression and produced a robust silencing of STN neurons as measured using whole-cell recording ex vivo. These results warrant further investigation to examine the therapeutic effects that STN HFS may have on relapse in humans with heroin addiction.
Figures


References
-
- Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H et al (2006). Pathological gambling in Parkinson's disease improves on chronic subthalamic nucleus stimulation. Movement Disord 21: 1941–1946. - PubMed
-
- Baunez C, Dias C, Cador M, Amalric M (2005). The subthalamic nucleus exerts opposite control on cocaine and 'natural' rewards. Nat Neurosci 8: 484–489. - PubMed
-
- Benazzouz A, Piallat B, Pollak P, Benabid AL (1995). Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett 189: 77–80. - PubMed
-
- Beurrier C, Bioulac B, Audin J, Hammond C (2001). High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

