Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance
- PMID: 27917898
- PMCID: PMC5137136
- DOI: 10.1038/srep38253
Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance
Abstract
The role of Sox2 in neurosensory development is not yet fully understood. Using mice with conditional Islet1-cre mediated deletion of Sox2, we explored the function of Sox2 in neurosensory development in a model with limited cell type diversification, the inner ear. In Sox2 conditional mutants, neurons initially appear to form normally, whereas late- differentiating neurons of the cochlear apex never form. Variable numbers of hair cells differentiate in the utricle, saccule, and cochlear base but sensory epithelium formation is completely absent in the apex and all three cristae of the semicircular canal ampullae. Hair cells differentiate only in sensory epithelia known or proposed to have a lineage relationship of neurons and hair cells. All initially formed neurons lacking hair cell targets die by apoptosis days after they project toward non-existing epithelia. Therefore, late neuronal development depends directly on Sox2 for differentiation and on the survival of hair cells, possibly derived from common neurosensory precursors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





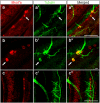
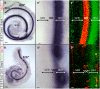
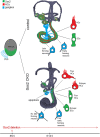
References
-
- Kondoh H. & Lovell-Badge R. Sox2: Biology and Role in Development and Disease. 3–15 (Elsevier, 2016).
-
- Reiprich S. & Wegner M. From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res 359, 111–124 (2015). - PubMed
-
- Telley L. et al. Sequential transcriptional waves direct the differentiation of newborn neurons in the mouse neocortex. Science 351, 1443–1446 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

