c-MET as a Potential Therapeutic Target in Ovarian Clear Cell Carcinoma
- PMID: 27917934
- PMCID: PMC5137074
- DOI: 10.1038/srep38502
c-MET as a Potential Therapeutic Target in Ovarian Clear Cell Carcinoma
Abstract
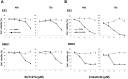
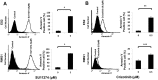
In this study, we investigated the therapeutic effects of c-MET inhibition in ovarian clear cell carcinoma (OCCC). Expression levels of c-MET in the epithelial ovarian cancers (EOCs) and normal ovarian tissues were evaluated using real-time PCR. To test the effects of c-MET inhibitors in OCCC cell lines, we performed MTT and apoptosis assays. We used Western blots to evaluate the expression of c-MET and its down-stream pathway. In vivo experiments were performed to test the effects of c-MET inhibitor on tumor growth in orthotopic mouse xenografts of OCCC cell line RMG1 and a patient-derived tumor xenograft (PDX) model of OCCC. c-MET expression was significantly greater in OCCCs compared with serous carcinomas and normal ovarian tissues (p < 0.001). In in vitro study, inhibition of c-MET using c-MET inhibitors (SU11274 or crizotinib) significantly decreased the proliferation, and increased the apoptosis of OCCC cells. SU11274 decreased expression of the p-c-MET proteins and blocked the phosphorylation of down-stream proteins Akt and Erk. Furthermore, SU11274 treatment significantly decreased the in vivo tumor weight in xenograft models of RMG1 cell and a PDX model for OCCC compared to control (p = 0.004 and p = 0.009, respectively).
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch 460, 237–249 (2012). - PubMed
-
- Ho C. M. et al.. Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol 94, 197–203 (2004). - PubMed
-
- Lee Y. Y. et al.. Prognosis of ovarian clear cell carcinoma compared to other histological subtypes: a meta-analysis. Gynecol Oncol 122, 541–547 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

