Nitric oxide donors for cervical ripening and induction of labour
- PMID: 27918616
- PMCID: PMC6463948
- DOI: 10.1002/14651858.CD006901.pub3
Nitric oxide donors for cervical ripening and induction of labour
Abstract
Background: Sometimes it is necessary to bring on labour artificially because of safety concerns for the mother or baby. This review is one of a series of reviews of methods of labour induction using a standardised protocol.
Objectives: To determine the effects of NO donors (isosorbide mononitrate (ISMN), isosorbide dinitrate (ISDN), nitroglycerin and sodium nitroprusside) for third trimester cervical ripening or induction of labour, in comparison with placebo or no treatment or other treatments from a predefined hierarchy.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register (15 August 2016) and the reference lists of trial reports.
Selection criteria: Clinical trials comparing NO donors for cervical ripening or labour induction with other methods listed above it on a predefined list of methods of labour induction. Interventions include NO donors (isosorbide mononitrate, isosorbide dinitrate, nitroglycerin and sodium nitroprusside) compared with other methods listed above it on a predefined list of methods of labour induction.
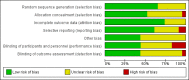
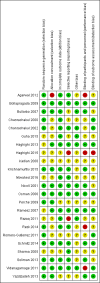
Data collection and analysis: This review is part of a series of reviews focusing on methods of induction of labour, based on a generic protocol. Three review authors independently assessed trials for inclusion, assessed risk of bias and extracted data. In this update, the quality of the evidence for the main comparison was assessed using the GRADE approach.
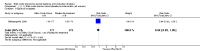
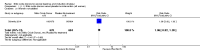
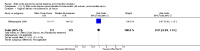
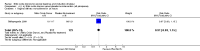
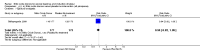
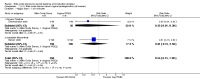
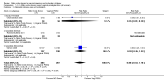
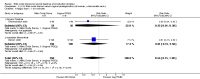
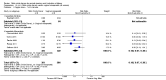
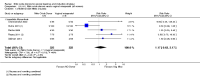
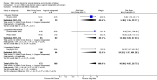
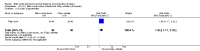
Main results: We included 23 trials (including a total of 4777 women). Included studies compared NO donors with placebo, vaginal prostaglandin E2 (PGE2), intracervical PGE2, vaginal misoprostol and intracervical Foley catheter. The majority of the included studies were assessed as being at low risk of bias. Nitric oxide versus placebo There was no evidence of a difference for any of the primary outcomes analysed: vaginal delivery not achieved in 24 hours (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.83 to 1.15; one trial, 238 women; low-quality evidence), uterine hyperstimulation with fetal heart rate (FHR) changes (RR 0.09, 95% CI 0.01 to 1.62; two trials, 300 women; low-quality evidence), caesarean section (RR 0.99, 95% CI 0.88 to 1.11; nine trials, 2624 women; moderate-quality evidence) or serious neonatal morbidity/perinatal death (average RR 1.61, 95% CI 0.08 to 33.26; two trials, 1712 women; low-quality evidence). There were no instances of serious maternal morbidity or death (one study reported this outcome).There was a reduction in an unfavourable cervix at 12 to 24 hours in women treated with NO donors (average RR 0.78, 95% CI 0.67 to 0.90; four trials, 762 women), and this difference was observed in both subgroups of standard release and slow release formulation. Women who received NO donors were less likely to experience uterine hyperstimulation without FHR rate changes (RR 0.05, 95% CI 0.00 to 0.80; one trial, 200 women), and more likely to experience side effects, including nausea, headache and vomiting. Nitric oxide donors versus vaginal prostaglandins There was no evidence of any difference between groups for uterine hyperstimulation with FHR changes or caesarean section (RR 0.97, 95% CI 0.78 to 1.21; three trials, 571 women). Serious neonatal morbidity and serious maternal morbidity were not reported. There were fewer women in the NO donor group who did not achieve a vaginal delivery within 24 hours (RR 0.63, 95% CI 0.47 to 0.86; one trial, 400 primiparae women). Nitric oxide donors versus intracervical prostaglandins One study reported a reduction in the number of women who had not achieved a vaginal delivery within 24 hours with NO donors (RR 0.63, 95% CI 0.47 to 0.86; one trial, 400 women). This result should be interpreted with caution as the information was extracted from an abstract only and a full report of the study is awaited. No differences were observed between groups for uterine hyperstimulation with FHR changes (RR 0.33, 95% CI 0.01 to 7.74; one trial, 42 women) or serious neonatal morbidity/perinatal death (RR 0.33, 95% CI 0.01 to 7.74; one trial, 42 women). Fewer women in the NO donor group underwent a caesarean section in comparison to women who received intracervical prostaglandins (RR 0.63, 95% CI 0.44 to 0.90; two trials, 442 women). No study reported on the outcome serious maternal morbidity or death. Nitric oxide donors versus vaginal misoprostol There was a reduction in the rate of uterine hyperstimulation with FHR changes with NO donors (RR 0.07, 95% CI 0.01 to 0.37; three trials, 281 women). There were no differences in caesarean section rates (RR 1.00, 95% CI 0.82 to 1.21; 761 women; six trials) and no cases of serious neonatal morbidity/perinatal death were reported. One study found that women in the NO donor group were more likely to not deliver within 24 hours (RR 5.33, 95% CI 1.62 to 17.55; one trial, 150 women). Serious maternal morbidity or death was not reported.In terms of secondary outcomes, there was an increase in cervix unchanged/unfavourable with NO (RR 3.43, 95% CI 2.07 to 5.66; two trials, 151 women) and an increase in the need for oxytocin augmentation with NO induction (RR 2.67, 95% CI 1.31 to 5.45; 7 trials; 767 women), although there was evidence of significant heterogeneity which could not be fully explained. Uterine hyperstimulation without FHR was lower in the NO group, as was meconium-stained liquor, Apgar score less than seven at five minutes and analgesia requirements. Nitric oxide donors versus intracervical catheter There was no evidence on any difference between the effects of NO and the use of a Foley catheter for induction of labour for caesarean section (RR 1.00, 95% CI 0.39 to 2.59; one trial, 80 women). No other primary outcomes were reported. One study of 75 participants did not contribute any data to the review.For all comparisons, women who received NO donors were more likely to experience side effects such as headache, nausea or vomiting.
Authors' conclusions: Available data suggests that NO donors can be a useful tool in the process of induction of labour causing the cervix to be more favourable in comparison to placebo. However, additional data are needed to assess the true impact of NO donors on all important labour process and delivery outcomes.
Conflict of interest statement
Arpita Ghosh: none known.
Katherine R Lattey: none known.
Anthony J Kelly: none known.
Figures








































































































































































































































































































































































Update of
-
Nitric oxide donors for cervical ripening and induction of labour.Cochrane Database Syst Rev. 2011 Jun 15;(6):CD006901. doi: 10.1002/14651858.CD006901.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Dec 05;12:CD006901. doi: 10.1002/14651858.CD006901.pub3. PMID: 21678363 Updated.
References
References to studies included in this review
Agarwal 2012 {published data only}
-
- Agarwal K, Batra A, Dabral A, Aggarwal A. Evaluation of isosorbide mononitrate for cervical ripening prior to induction of labor for postdated pregnancy in an outpatient setting. International Journal of Gynecology and Obstetrics 2012;118(3):205‐9. - PubMed
Bollapragada 2009 {published data only}
-
- Bollapragada S, Mackenzie F, Norrie J, Petrou S, Reid M, Greer I, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour ‐ clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy and Childbirth 2006;6:25. - PMC - PubMed
-
- Bollapragada SS, MacKenzie F, Norrie J, Petrou S, Reid M, Greer IA, et al. Randomized placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour ‐ clinical trial with analyses of efficacy, cost effectiveness and acceptability. The IMOP study [abstract]. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S22. - PubMed
-
- Bollapragada SS, MacKenzie F, Norrie JD, Eddama O, Petrou S, Reid M, et al. Randomised placebo‐controlled trial of outpatient (at home) cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour‐clinical trial with analyses of efficacy and acceptability. The IMOP study. BJOG: an international journal of obstetrics and gynaecology 2009;116(9):1185‐95. - PubMed
-
- Eddama O, Petrou S, Schroeder L, Bollapragada SS, Mackenzie F, Norrie J, et al. The cost‐effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG: an international journal of obstetrics and gynaecology 2009;116(9):1196‐203. - PubMed
-
- Norman J. Pharmacokinetics of nitric oxide donors administered per vaginam in the third trimester of pregnancy. National Research Register (www.controlled‐trials.com) (accessed 26 July 2001).
Bullarbo 2007 {published data only}
-
- Bullarbo M, Eriksson O, Andersch B, Granstrom L, Norstrom A, Erkerhovd E. Outpatient vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening and labor induction postterm: a randomized controlled study. American Journal of Obstetrics and Gynecology 2007;196:50.e1‐50.e5. - PubMed
-
- Bullarbo M, Norstrom A, Andersch B, Ekerhovd E. Isosorbide mononitrate induces increased cervical expression of cyclooxygenase‐2, but not of cyclooxygenase‐1, at term. European Journal of Obstetrics & Gynecology and Reproductive Biology 2007;130(2):160‐4. - PubMed
Chanrachakul 2000 {published data only}
-
- Chanrachakul B, Herabutya Y, Punyavachira P. Potential efficacy of nitric oxide for cervical ripening on pregnancy at term. International Journal of Gynecology & Obstetrics 2000;71:217‐9. - PubMed
-
- Chanrachakul B, Herabutya Y, Punyavachira P. Randomised comparison of glyceryl trinitrate and prostaglandin E2 for cervical ripening at term. Obstetrics and Gynecology 2000;96:549‐53. - PubMed
-
- Chanrachakul B, Herbutya Y. Phase II to determine the potential efficacy and safety of nitric oxide for cervical ripening in pregnancy at term. XVI FIGO World Congress of Obstetrics & Gynecology (Book 4); 2000 Sept 3‐8; Washington DC, USA. 2000:68‐9.
Chanrachakul 2002 {published data only}
-
- Chanrachakul B, Herabutya Y, Punyavachira P. Randomized trial of isosorbide mononitrate versus misoprostol for cervical ripening at term. International Journal of Gynecology & Obstetrics 2002;78:139‐45. - PubMed
Guha 2015 {published data only}
-
- Guha K, Fatema A, Biswas PK, Haque E. Isosorbide mononitrate versus misoprostol for cervical ripening and induction of labour at term. Mymensingh Medical Journal : Mmj 2015;24(2):346‐51. - PubMed
Haghighi 2013 {published data only}
-
- Haghighi L, Homam H, Raoofi Z, Najmi Z. Intravaginal isosorbide dinitrate or misoprostol for cervical ripening prior to induction of labour: A randomised controlled trial. Journal of Obstetrics and Gynaecology 2013;33(3):272‐6. - PubMed
Haghighi 2015 {published data only}
-
- Moukhah S, Ahmadi F. Preinduction cervical ripening using oral and vaginal isosorbide dinitrate in patients with term pregnancy: A randomized clinical trial. Koomesh 2016;17(4):863‐70.
Kadian 2008 {published data only}
-
- Kadian ND. Comparison of nitric oxide donor isosorbide dinitrate (IDN) and dinoprostone for cervical ripening before induction of labor at term. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):76.
Krishnamurthy 2015 {published data only}
Movahed 2016 {published data only}
-
- Movahed F, Seyed E, Pakniat H, Iranipour M, Yazdi Z. Comparison of the effects of transcervical catheter, laminaria and isosorbide mononitrate on cervical ripening. Journal of Babol University of Medical Sciences 2016;18(3):19‐24.
Nicoll 2001 {published data only}
-
- Nicoll AE, Mackenzie F, Greer IA, Norman JE. Vaginal application of the nitric oxide donor isosorbide mononitrate for preinduction cervical ripening: a randomized controlled trial to determine effects on maternal and fetal hemodynamics. American Journal of Obstetrics and Gynecology 2001;184:958‐64. - PubMed
Osman 2006 {published data only}
-
- Osman I, MacKenzie F, Norrie J, Murray HM, Greer IA, Norman JE. The "PRIM" study: a randomized comparison of prostaglandin E2 gel with the nitric oxide donor isosorbide mononitrate for cervical ripening before the induction of labor at term. American Journal of Obstetrics and Gynecology 2006;194(4):1012‐21. - PubMed
-
- Osman I, MacKenzie F, Norrie J, Murray HM, Greer IA, Norman JE. The 'PRIM' study ‐ a randomised comparison of prostaglandin with isosorbide mononitrate for pre‐induction cervical ripening at term [abstract]. BJOG: an international journal of obstetrics and gynaecology 2005;112(4):512.
-
- Osman I, Mackenzie F, Norrie J, Greer A, Norman JE. The 'PRIM' study ‐ a randomised comparison of prostaglandin with isosorbide mononitrate for preinduction cervical ripening at term [abstract]. Journal of Obstetrics and Gynaecology 2004;24(Suppl 1):S67.
-
- Osman I, Norman J, Mackenzie F, Murray H, Norrie J, Greer I. The "PRIM" study: a randomised comparison of prostaglandin E2 gel with the nitric oxide donor isosorbide mononitrate for cervical ripening prior to the induction of labour at term [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S184. - PubMed
Perche 2009 {published data only}
-
- Perche S, Guerra M, Reyna E, Hidalgo M, Santos J, Mejia J, et al. Vaginal isosorbide mononitrate or misoprostol for cervical ripening in term pregnancies. Clinica e Investigacion en Ginecologia y Obstetricia 2009;36(6):203‐8.
Rameez 2007 {published data only}
-
- Rameez MF, Goonewardene IM. Nitric oxide donor isosorbide mononitrate for pre‐induction cervical ripening at 41 weeks' gestation: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2007;33(4):452‐6. - PubMed
Razaq 2011 {published data only}
-
- Razaq A. Isosorbide mononitrate versus misoprostol for cervical ripening. Al‐Kindy College Medical Journal 2011;8(1):69‐74.
Rezk 2014 {published data only}
-
- Rezk M, Sanad Z, Dawood R, Masood A, Emarh M, Halaby AA. Intracervical foley catheter versus vaginal isosorbid mononitrate for induction of labor in women with previous one cesarean section. Journal of Clinical Gynecology and Obstetrics 2014;3(2):55‐61.
Romero‐Gutierrez 2011 {published data only}
-
- Romero‐Gutierrez G, Gonzalez OEB, Ponce‐Ponce de Leon A. Isosorbide and dinoprostone for induction of labor [Isosorbide y dinoprostona en induccion del trabajo de parto]. Ginecologia y Obstetricia de Mexico 2011;79(5):285‐91. - PubMed
Schmitz 2014 {published data only}
-
- Schmitz T, Closset E, Fuchs F, Maillard F, Rozenberg P, Anselem O, et al. Outpatient cervical ripening with nitric oxide (NO) donors for prolonged pregnancy in nullipara: the NOCETER randomized, multicentre, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S19.
-
- Schmitz T, Fuchs F, Closset E, Rozenberg P, Winer N, Perrotin F, et al. Outpatient cervical ripening by nitric oxide donors for prolonged pregnancy: a randomized controlled trial. Obstetrics and Gynecology 2014;124(6):1089‐97. - PubMed
Sharma 2005 {published data only}
-
- Sharma Y, Kumar S, Mittal S, Misra R, Dadhwal V. Evaluation of glyceryl trinitrate, misoprostol, and prostaglandin E2 gel for preinduction cervical ripening in term pregnancy. Journal of Obstetrics and Gynaecology Research 2005;31(3):210‐5. - PubMed
Soliman 2013 {published data only}
-
- Soliman AT. A comparison of isosorbide mononitrate, misoprostol, and combination therapy for preinduction cervical ripening at term: a randomized controlled trial. Tanta Medical Journal 2013;41(4):310‐7.
Vidanagamage 2011 {published data only}
-
- Vidanagamage RS, Goonewardene IM. The efficacy of two different doses of vaginal isosorbide mononitrate in pre induction cervical ripening: a double blind randomised controlled trial. Ceylon Medical Journal 2011;56(3):91‐100. - PubMed
Yazdizadeh 2013 {published data only}
-
- Yazdizadeh H, Abedi P, Najar S, Angali KA. The impact of isosorbide mononitrate on cervical ripening and labor induction in primiparous women with term pregnancy: A double‐blind, randomized, controlled trial. Iranian Journal of Nursing and Midwifery Research 2013; Vol. 18, issue 3:246‐50. - PMC - PubMed
References to studies excluded from this review
Abdellah 2011 {published data only}
-
- Abdellah MS, Hussien M, AboAlhassan A. Intravaginal administration of isosorbide mononitrate and misoprostol for cervical ripening and induction of labour: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;284(1):25‐30. - PubMed
Ahmed 2014 {published data only}
-
- Ahmed WAS, Ahmed MR, Madny EH, Mohamed RM, Elshahat AM. A comparison between two different doses of vaginal isosorbide mononitrate versus misoprostol in pre‐induction cervical ripening at term: a randomized controlled study. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):150.
Bates 2003 {published data only}
-
- Bates CD, Nicoll AE, Mullen AB, Mackenzie F, Thomson AJ, Norman JE. Serum profile of isosorbide mononitrate after vaginal administration in the third trimester. BJOG: an international journal of obstetrics and gynaecology 2003;110:64‐7. - PubMed
Collingham 2010 {published data only}
-
- Collingham J, Fuh K, Caughey A, Pullen K, Lyell D, Druzin M, et al. Randomized clinical trial of cervical ripening and labor induction using oral misoprostol with or without intravaginal isosorbide mononitrate. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S57.
-
- Collingham JP, Fuh KC, Caughey AB, Pullen KM, Lyell DJ, El‐Sayed YY. Oral misoprostol and vaginal isosorbide mononitrate for labor induction: a randomized controlled trial. Obstetrics & Gynecology 2010;116(1):121‐6. - PubMed
Ekerhovd 2003 {published data only}
-
- Ekerhovd E, Bullarbo M, Andersch B, Norstrom A. Vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening at term: a randomized controlled study. American Journal of Obstetrics and Gynecology 2003;189:1692‐7. - PubMed
El‐Khayat 2016 {published data only}
-
- El‐Khayat W, Alelaiw H, El‐Kateb A, Elsemary A. Comparing vaginal misoprostol versus foley catheter plus vaginal isosorbide mononitrate for labor induction. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(3):487‐92. - PubMed
Habib 2008 {published data only}
-
- Habib SM, Emam SS, Saber AS. Outpatient cervical ripening with nitric oxide donor isosorbide mononitrate prior to induction of labor. International Journal of Gynecology & Obstetrics 2008;101(1):57‐61. - PubMed
Helal 2004 {published data only}
-
- Helal AMM, Abd El‐Razek MME, Ahmed RE. Randomized double‐blind controlled study to determine the effect of vaginally applied nitric oxide donor (isosorbide mononitrate) for preinduction cervical ripening on maternal & fetal hemodynamics and the ripening of the cervix. El‐Minia Medical Bulletin 2004;15(1):32‐42.
Moghtadaei 2007 {published data only}
-
- Moghtadaei P. A randomized trial comparing outpatient vaginal isosorbide‐mononitrate versus extra‐amnion saline infusion with concurrent oxytocin for cervical ripening and labor induction in nulliparous women. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S103.
-
- Moghtadei P, Sardari F. A randomized trial comparing outpatient vaginal isosorbide mononitrate versus extra‐amnion saline infusion with concurrent oxytocin for cervical ripening and labor induction in nulliparous women. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):77.
Nunes 2006 {published data only}
-
- Nunes FP, Campos AP, Pedroso SR, Leite CF, Avillez TP, Rodrigues RD, et al. Intravaginal glyceryl trinitrate and dinoprostone for cervical ripening and induction of labor. American Journal of Obstetrics and Gynecology 2006;194(4):1022‐6. - PubMed
Vaisanen‐Tommiska 2008 {published data only}
-
- Vaisanen‐Tommiska M, Mikkola T, Ylikorkala O. Vaginal nitro induces cervical nitric oxide release in women postterm. 36th Nordic Congress of Obstetrics and Gynecology; 2008 June 14‐17; Reykjavik, Iceland. 2008:123.
Wolfler 2006 {published data only}
-
- Wolfler MM, Facchinetti F, Venturini P, Huber A, Helmer H, Husslein P, et al. Induction of labor at term using isosorbide mononitrate simultaneously with dinoprostone compared to dinoprostone treatment alone: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2006;195(6):1617‐22. - PubMed
Ziard 2012 {published data only}
-
- Ziard MH, Goonewardene IMR. Efficacy of two different treatment regimens of vaginal nitric oxide donor (iso‐sorbidemononitrate) used for pre‐induction cervical ripening. Sri Lanka Journal of Obstetrics and Gynaecology 2012;34(Suppl 1):81, Abstract no: OP 13.
References to studies awaiting assessment
Ghanaie 2013 {published data only}
-
- Ghanaie MM, Mirblouk F, Godarzi R, Shakiba M. Effect of outpatient isosorbide mononitrate on success of labor induction. Journal of Babol University of Medical Sciences 2013;15(2):12‐7.
Additional references
Alfirevic 2009
Alfirevic 2014
Arnold 1977
Boulvain 2005
Boulvain 2008
Boulvain 2016
Bricker 2000
Bryman 1986
-
- Bryman I, Norström A, Lindblom B. Influence of prostaglandins and adrenoceptor agonists on contractile activity in the human cervix at term. Obstetrics & Gynecology 1986;67:574‐8. - PubMed
Buhimschi 1995
-
- Buhimschi I, Yallampalli C, Dong Y. Involvement of nitric‐oxide‐cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. American Journal of Obstetrics and Gynecology 1995;172:1577‐84. - PubMed
Calder 1998
-
- Calder AA. Nitric oxide: another factor in cervical ripening. Human Reproduction 1998;13:250‐1. - PubMed
Chwalisz 1998
-
- Chwalisz K, Garfield RE. Nitric oxide as the final mediator of cervical ripening. Human Reproduction 1998;13:245‐52. - PubMed
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. - PubMed
Ekerhord 1998
-
- Ekerhord E, Brannstrom M, Delbo D, Norstrom A. Nitric oxide mediated inhibition of contractile activity in the human cervix. Molecular Human Reproduction 1998;4:915‐20. - PubMed
French 2001
Hapangama 2009
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2003
Hofmeyr 2009
-
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074] - DOI
Howarth 2001
Hutton 2001
Izurni 1993
-
- Izurni H, Yallampall C, Carfield R. Gestational changes in L‐arginine‐induced relaxation of pregnant rat and human myometrial smooth muscle. American Journal of Obstetrics and Gynecology 1993;169(5):1327‐37. - PubMed
Jozwiak 2012
Kavanagh 2001
Kavanagh 2005
Kavanagh 2006a
Kavanagh 2006b
Kelly 2001a
Kelly 2013b
Lees 1994
-
- Lees C, Campbell S, Jaunaax E, Brown R, Ramsey B, Cibb D. Arrest of pre‐term labour and prolongation of gestation with glyceryl trinitrate, a nitric oxide donor. Lancet 1994;343:1325‐6. - PubMed
Luckas 2000
Muzonzini 2004
Norman 1997
-
- Norman J, Ward I, Martin W, Cameron A, McGrath J, Creer I. Effects of cGMP and the nitric oxide donors glyceryl trinitrate and sodium nitroprusside on contractions in vitro of isolated myometrial tissue from pregnant women. Journal of Repoductive Fertility 1997;110:249‐54. - PubMed
Qing 1996
-
- Qing S, Bier H, Garfiled R. Local application of nitric oxide (NO) donors induces cervical ripening. Journal of the Society of Gynaecological Investigation 1996;3:462.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2003
Smith 2013
Telfer 1995
-
- Telfer J, Lyall F, Norman J. Identification of nitric oxide synthase in human uterus. Human Reproduction 1995;10:19‐23. - PubMed
Thomas 2001
Thomas 2014
Thompson 1997
-
- Thompson A, Luman C, Cameron A. Nitric oxide donors induce ripening of the human uterine cervix: a randomised control trial. British Journal of Obstetrics and Gynaecology 1997;104:1054‐7. - PubMed
Thompson 1998
-
- Thompson A, Lunan C, Ledingham M, Howat R, Cameron I, Greer I. Randomised trial of nitric oxide donors versus prostaglandins for cervical ripening before first trimester termination of pregnancy. Lancet 1998;352:1093‐6. - PubMed
References to other published versions of this review
Kelly 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

