Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans
- PMID: 27919065
- PMCID: PMC5361225
- DOI: 10.1038/nature20789
Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans
Erratum in
-
Corrigendum: Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans.Nature. 2017 Jul 27;547(7664):476. doi: 10.1038/nature23313. Epub 2017 Jul 12. Nature. 2017. PMID: 28700577
Abstract
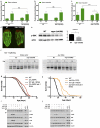
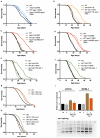
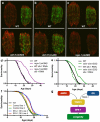
Ageing is driven by a loss of transcriptional and protein homeostasis and is the key risk factor for multiple chronic diseases. Interventions that attenuate or reverse systemic dysfunction associated with age therefore have the potential to reduce overall disease risk in the elderly. Precursor mRNA (pre-mRNA) splicing is a fundamental link between gene expression and the proteome, and deregulation of the splicing machinery is linked to several age-related chronic illnesses. However, the role of splicing homeostasis in healthy ageing remains unclear. Here we demonstrate that pre-mRNA splicing homeostasis is a biomarker and predictor of life expectancy in Caenorhabditis elegans. Using transcriptomics and in-depth splicing analysis in young and old animals fed ad libitum or subjected to dietary restriction, we find defects in global pre-mRNA splicing with age that are reduced by dietary restriction via splicing factor 1 (SFA-1; the C. elegans homologue of SF1, also known as branchpoint binding protein, BBP). We show that SFA-1 is specifically required for lifespan extension by dietary restriction and by modulation of the TORC1 pathway components AMPK, RAGA-1 and RSKS-1/S6 kinase. We also demonstrate that overexpression of SFA-1 is sufficient to extend lifespan. Together, these data demonstrate a role for RNA splicing homeostasis in dietary restriction longevity and suggest that modulation of specific spliceosome components may prolong healthy ageing.
Figures






Comment in
-
Making a splash with splicing.Cell Res. 2017 Apr;27(4):457-458. doi: 10.1038/cr.2017.24. Epub 2017 Feb 24. Cell Res. 2017. PMID: 28233771 Free PMC article.

