Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions
- PMID: 27919268
- PMCID: PMC5137211
- DOI: 10.1186/s12989-016-0171-3
Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions
Abstract
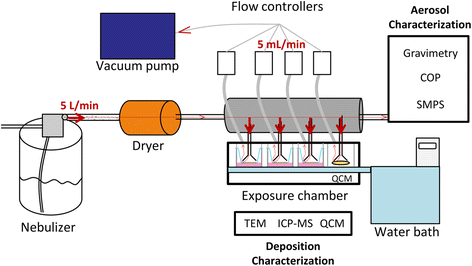
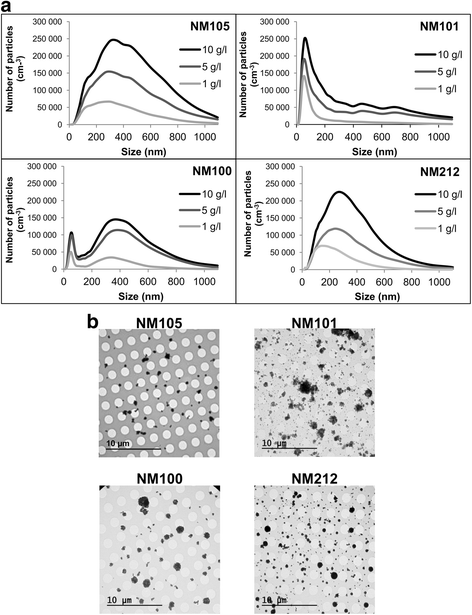
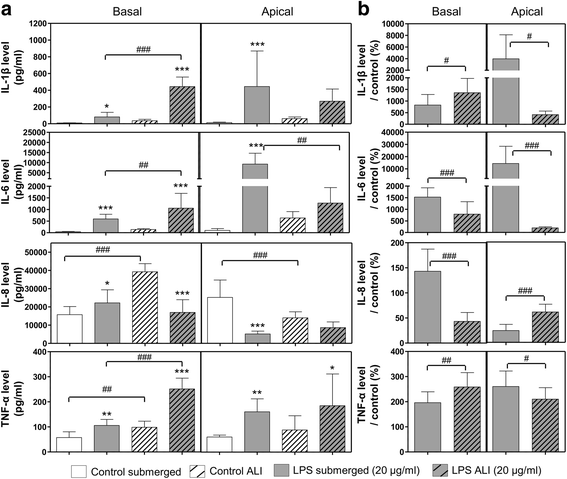
Background: Recently, much progress has been made to develop more physiologic in vitro models of the respiratory system and improve in vitro simulation of particle exposure through inhalation. Nevertheless, the field of nanotoxicology still suffers from a lack of relevant in vitro models and exposure methods to predict accurately the effects observed in vivo, especially after respiratory exposure. In this context, the aim of our study was to evaluate if exposing pulmonary cells at the air-liquid interface to aerosols of inhalable and poorly soluble nanomaterials generates different toxicity patterns and/or biological activation levels compared to classic submerged exposures to suspensions. Three nano-TiO2 and one nano-CeO2 were used. An exposure system was set up using VitroCell® devices to expose pulmonary cells at the air-liquid interface to aerosols. A549 alveolar cells in monocultures or in co-cultures with THP-1 macrophages were exposed to aerosols in inserts or to suspensions in inserts and in plates. Submerged exposures in inserts were performed, using similar culture conditions and exposure kinetics to the air-liquid interface, to provide accurate comparisons between the methods. Exposure in plates using classical culture and exposure conditions was performed to provide comparable results with classical submerged exposure studies. The biological activity of the cells (inflammation, cell viability, oxidative stress) was assessed at 24 h and comparisons of the nanomaterial toxicities between exposure methods were performed.
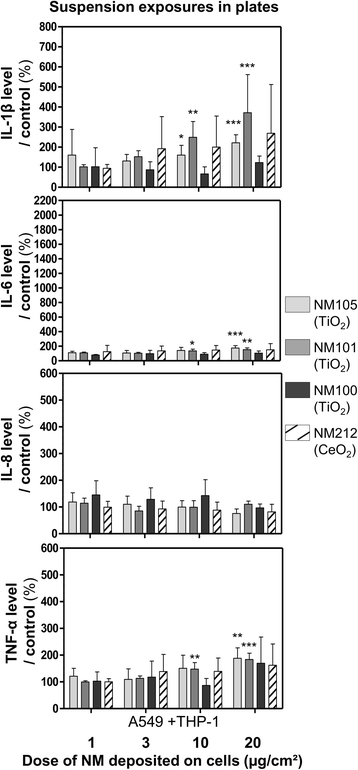
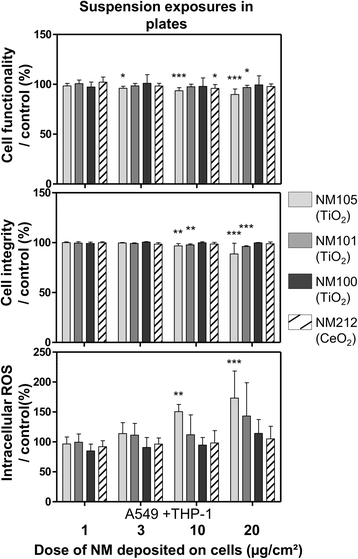
Results: Deposited doses of nanomaterials achieved using our aerosol exposure system were sufficient to observe adverse effects. Co-cultures were more sensitive than monocultures and biological responses were usually observed at lower doses at the air-liquid interface than in submerged conditions. Nevertheless, the general ranking of the nanomaterials according to their toxicity was similar across the different exposure methods used.
Conclusions: We showed that exposure of cells at the air-liquid interface represents a valid and sensitive method to assess the toxicity of several poorly soluble nanomaterials. We underlined the importance of the cellular model used and offer the possibility to deal with low deposition doses by using more sensitive and physiologic cellular models. This brings perspectives towards the use of relevant in vitro methods of exposure to assess nanomaterial toxicity.
Keywords: Air-liquid interface; Alveolar cells; Co-culture; In vitro; Nanomaterials; Submerged conditions; Toxicity.
Figures





References
-
- Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res. 2012;14(9). doi: Artn 1109 10.1007/S11051-012-1109-9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

