Patient-derived xenografts: a relevant preclinical model for drug development
- PMID: 27919280
- PMCID: PMC5139018
- DOI: 10.1186/s13046-016-0462-4
Patient-derived xenografts: a relevant preclinical model for drug development
Abstract
Identifying appropriate preclinical cancer models remains a major challenge in increasing the efficiency of drug development. A potential strategy to improve patient outcomes could be selecting the 'right' treatment in preclinical studies performed in patient-derived xenografts (PDXs) obtained by direct implants of surgically resected tumours in mice. These models maintain morphological similarities and recapitulate molecular profiling of the original tumours, thus representing a useful tool in evaluating anticancer drug response. In this review, we will present the state-of-art use of PDXs as a reliable strategy to predict clinical findings. The main advantages and limitations will also be discussed.
Keywords: Immunosuppressed mice; Orthotopic; Patient-derived-xenografts; Predictive value; Subcutaneous; Tumor heterogeneity.
Figures
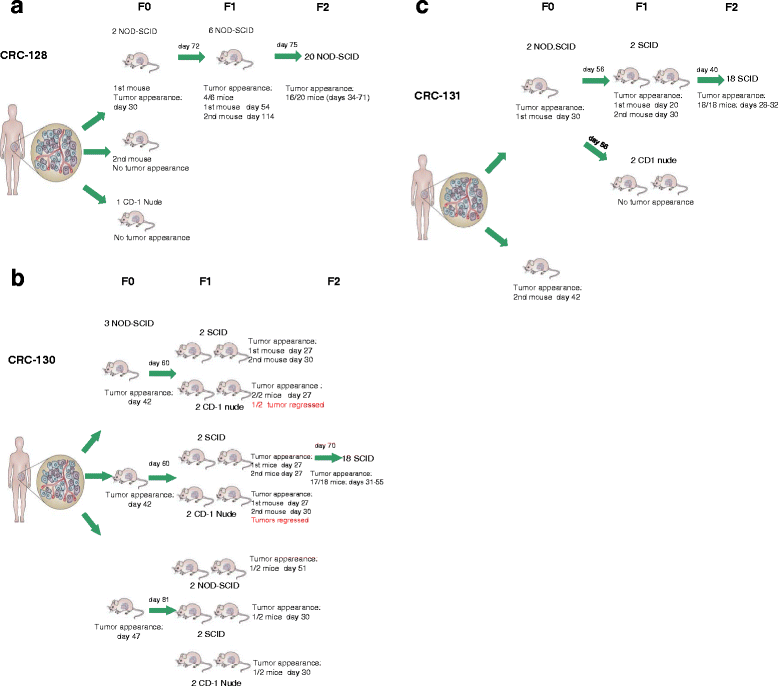
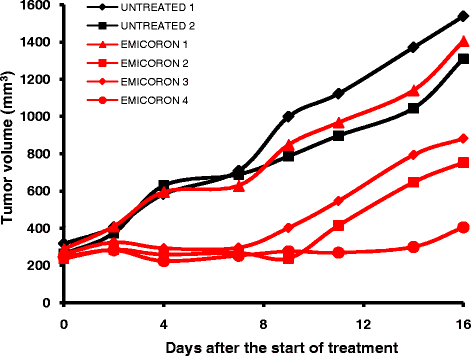
References
-
- Kirschbaum A, Geisse NC, Sister TJ, Meyer LM. Effect of certain folic acid antagonists on transplanted myeloid and lymphoid leukemias of the F strain of mice. Cancer Res. 1950;10:762–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

