Drosophila neprilysins control insulin signaling and food intake via cleavage of regulatory peptides
- PMID: 27919317
- PMCID: PMC5140268
- DOI: 10.7554/eLife.19430
Drosophila neprilysins control insulin signaling and food intake via cleavage of regulatory peptides
Abstract
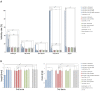
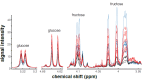
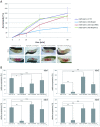
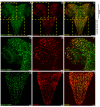
Insulin and IGF signaling are critical to numerous developmental and physiological processes, with perturbations being pathognomonic of various diseases, including diabetes. Although the functional roles of the respective signaling pathways have been extensively studied, the control of insulin production and release is only partially understood. Herein, we show that in Drosophila expression of insulin-like peptides is regulated by neprilysin activity. Concomitant phenotypes of altered neprilysin expression included impaired food intake, reduced body size, and characteristic changes in the metabolite composition. Ectopic expression of a catalytically inactive mutant did not elicit any of the phenotypes, which confirms abnormal peptide hydrolysis as a causative factor. A screen for corresponding substrates of the neprilysin identified distinct peptides that regulate insulin-like peptide expression, feeding behavior, or both. The high functional conservation of neprilysins and their substrates renders the characterized principles applicable to numerous species, including higher eukaryotes and humans.
Keywords: D. melanogaster; developmental biology; epidemiology; feeding behavior; global health; insulin expression; insulin signaling; metallopeptidase; neprilysin; peptide metabolism; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





References
-
- Baggerman G, Boonen K, Verleyen P, De Loof A, Schoofs L. Peptidomic analysis of the larval Drosophila melanogaster central nervous system by two-dimensional capillary liquid chromatography quadrupole time-of-flight mass spectrometry. Journal of Mass Spectrometry. 2005;40:250–260. doi: 10.1002/jms.744. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

