Neutralization Takes Precedence Over IgG or IgA Isotype-related Functions in Mucosal HIV-1 Antibody-mediated Protection
- PMID: 27919754
- PMCID: PMC5161443
- DOI: 10.1016/j.ebiom.2016.11.024
Neutralization Takes Precedence Over IgG or IgA Isotype-related Functions in Mucosal HIV-1 Antibody-mediated Protection
Abstract
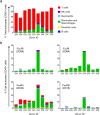
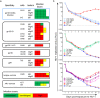
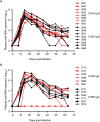
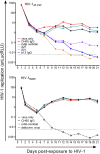
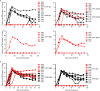
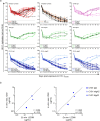
HIV-1 infection occurs primarily through mucosal transmission. Application of biologically relevant mucosal models can advance understanding of the functional properties of antibodies that mediate HIV protection, thereby guiding antibody-based vaccine development. Here, we employed a human ex vivo vaginal HIV-1 infection model and a rhesus macaque in vivo intrarectal SHIV challenge model to probe the protective capacity of monoclonal broadly-neutralizing (bnAb) and non-neutralizing Abs (nnAbs) that were functionally modified by isotype switching. For human vaginal explants, we developed a replication-competent, secreted NanoLuc reporter virus system and showed that CD4 binding site bnAbs b12 IgG1 and CH31 IgG1 and IgA2 isoforms potently blocked HIV-1JR-CSF and HIV-1Bal26 infection. However, IgG1 and IgA nnAbs, either alone or together, did not inhibit infection despite the presence of FcR-expressing effector cells in the tissue. In macaques, the CH31 IgG1 and IgA2 isoforms infused before high-dose SHIV challenge were completely to partially protective, respectively, while nnAbs (CH54 IgG1 and CH38 mIgA2) were non-protective. Importantly, in both mucosal models IgG1 isotype bnAbs were more protective than the IgA2 isotypes, attributable in part to greater neutralization activity of the IgG1 variants. These findings underscore the importance of potent bnAb induction as a primary goal of HIV-1 vaccine development.
Keywords: Antibodies; HIV-1; IgA; IgG; Mucosal immunology; Neutralizing antibodies; Non-human primate rectal challenge model; Vaginal explants.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Ahmed Z., Kawamura T., Shimada S., Piguet V. The role of human dendritic cells in HIV-1 infection. J. Invest. Dermatol. 2015;135:1225–1233. - PubMed
-
- Barouch D.H., Whitney J.B., Moldt B., Klein F., Oliveira T.Y., Liu J., Stephenson K.E., Chang H.W., Shekhar K., Gupta S., Nkolola J.P., Seaman M.S., Smith K.M., Borducchi E.N., Cabral C., Smith J.Y., Blackmore S., Sanisetty S., Perry J.R., Beck M., Lewis M.G., Rinaldi W., Chakraborty A.K., Poignard P., Nussenzweig M.C., Burton D.R. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. - PMC - PubMed
-
- Barouch D.H., Alter G., Broge T., Linde C., Ackerman M.E., Brown E.P., Borducchi E.N., Smith K.M., Nkolola J.P., Liu J., Shields J., Parenteau L., Whitney J.B., Abbink P., Ng'ang'a D.M., Seaman M.S., Lavine C.L., Perry J.R., Li W., Colantonio A.D., Lewis M.G., Chen B., Wenschuh H., Reimer U., Piatak M., Lifson J.D., Handley S.A., Virgin H.W., Koutsoukos M., Lorin C., Voss G., Weijtens M., Pau M.G., Schuitemaker H. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349:320–324. - PMC - PubMed
-
- Barouch D.H., Ghneim K., Bosche W.J., Li Y., Berkemeier B., Hull M., Bhattacharyya S., Cameron M., Liu J., Smith K., Borducchi E., Cabral C., Peter L., Brinkman A., Shetty M., Li H., Gittens C., Baker C., Wagner W., Lewis M.G., Colantonio A., Kang H.J., Li W., Lifson J.D., Piatak M., Jr., Sekaly R.P. Rapid Inflammasome activation following mucosal SIV infection of rhesus monkeys. Cell. 2016;165:656–667. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

